Constitutive systemic inflammation in Shwachman-Diamond Syndrome
- PMID: 40021961
- PMCID: PMC11869671
- DOI: 10.1186/s10020-025-01133-5
Constitutive systemic inflammation in Shwachman-Diamond Syndrome
Abstract
Background and purpose: Shwachman-Diamond Syndrome (SDS) is an autosomal recessive disease belonging to the inherited bone marrow failure syndromes and characterized by hypocellular bone marrow, exocrine pancreatic insufficiency, and skeletal abnormalities. SDS is associated with increased risk of developing myelodysplastic syndrome (MDS) and/or acute myeloid leukemia (AML). Although SDS is not primarily considered an inflammatory disorder, some of the associated conditions (e.g., neutropenia, pancreatitis and bone marrow dysfunction) may involve inflammation or immune system dysfunctions. We have already demonstrated that signal transducer and activator of transcription (STAT)-3 and mammalian target of rapamycin (mTOR) were hyperactivated and associated with elevated IL-6 levels in SDS leukocytes. In this study, we analyzed the level of phosphoproteins involved in STAT3 and mTOR pathways in SDS lymphoblastoid cells (LCLs) and the secretomic profile of soluble pro-inflammatory mediators in SDS plasma and LCLs in order to investigate the systemic inflammation in these patients and relative pathways.
Methods: Twenty-six SDS patients and seven healthy donors of comparable age were recruited during the programmed follow-up visits for clinical evaluation at the Verona Cystic Fibrosis Center Human. The obtained samples (plasma and/or LCLs) were analyzed for: phosphoproteins, cytokines, chemokines and growth factors levels by Bio-plex technology; microRNAs profiling by next generation sequencing (NGS) and microRNAs expression validation by Real Time-PCR (RT-PCR) and droplet digital PCR (ddPCR) .
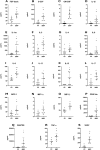
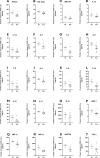
Results: We demonstrated dysregulation of ERK1/2 and AKT phosphoproteins in SDS, as their involvement in the hyperactivation of the STAT3 and mTOR pathways confirmed the interplay of these pathways in SDS pathophysiology. However, both these signaling pathways are strongly influenced by the inflammatory environment. Here, we reported that SDS is characterized by elevated plasma levels of several soluble proinflammatory mediators. In vitro experiments show that these pro-inflammatory genes are closely correlated with STAT3/mTOR pathway activation. In addition, we found that miR-181a-3p is down-regulated in SDS. Since this miRNA acts as a regulator of several pro-inflammatory pathways such as STAT3 and ERK1/2, its down-regulation may be a driver of the constitutive inflammation observed in SDS patients.
Conclusions: The results obtained in this study shed light on the complex pathogenetic mechanism underlying bone marrow failure and leukemogenesis in SDS, suggesting the need for anti-inflammatory therapies for SDS patients.
Keywords: Acute myeloid leukemia; Inflammation; Shwachman-Diamond Syndrome; mTOR.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Azienda Ospedaliera Universitaria Integrata (Verona, Italy: approval No. 4182 CESC). Informed consent was obtained from participants or their legal representatives. Consent for publication: All the authors agree to publish. Competing interests: The authors declare no competing interests.
Figures




References
-
- Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40(1):139–46. 10.1042/BST20110609. - PubMed
-
- Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. 10.1016/j.ctrv.2017.08.004. - PubMed
-
- Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307(1):97–101. 10.1016/0014-5793(92)80909-z. - PubMed
-
- Bakouny Z, Choueiri TK. IL-8 and cancer prognosis on immunotherapy. Nat Med. 2020;26(5):650–1. 10.1038/s41591-020-0873-9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

