Significant correlations of upregulated MPO expression with cytokine imbalance in ankylosing spondylitis patients and the inhibitory effect mediated by mesenchymal stem cells
- PMID: 40022014
- PMCID: PMC11871679
- DOI: 10.1186/s12891-025-08458-6
Significant correlations of upregulated MPO expression with cytokine imbalance in ankylosing spondylitis patients and the inhibitory effect mediated by mesenchymal stem cells
Abstract
Background: Little is known regarding both the role of myeloperoxidase (MPO) and the impact of mesenchymal stem cells (MSCs) on inflammatory and immune responses in ankylosing spondylitis (AS). This study is aimed to explore the role of MPO and the regulatory effect of umbilical cord-derived MSCs on MPO expression in monocytes in AS.
Methods: MPO mRNA expression in the peripheral blood mononuclear cells (PBMCs) was detected by Real-time PCR. Cytokines including IL-2, IFN-γ, IL-17 A, IL-4, IL-10, IL-6 and TNF-α were determined by flow cytometry. A co-culture system was established by culturing THP-1 cells with MSCs at a ratio of 5:1.
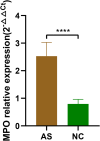
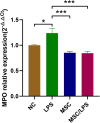
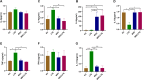
Results: Increased mRNA expression of MPO was observed in PBMCs of AS patients compared to healthy controls (P < 0.05). The mRNA expression of MPO was positively associated with C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (P < 0.05) in AS. Besides, the levels of IL-2, IL-10, IFN-γ, IL-17 A, IL-4, IL-6, TNF-α in plasma were notably increased in AS (P < 0.05). Positive correlations between MPO expression and IL-2, IFN -γ, IL-4, TNF-α as well as IL-6 were demonstrated in AS (P < 0.05). Furthermore, MSCs remarkably suppressed the mRNA expression of MPO along with the secretion of IL-17 A and TNF-α, but promoted IL-10 generation in monocytes.
Conclusion: MPO expression is significantly upregulated and correlates with cytokine imbalance in AS. It may serve as a valuable immunotherapeutic target for AS. MSCs can significantly inhibit monocyte-mediated inflammatory response potentially by downregulating MPO in monocytes.
Keywords: Ankylosing spondylitis; Cytokines; Mesenchymal stem cells; Myeloperoxidase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the Medical Ethics Committee of Weifang People’s Hospital, Shandong Second Medical University (date: 12.12.2021, No. 2021YX074). All patients provided informed consent, and the study was conducted in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures



Similar articles
-
The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients.J Neurochem. 2014 Oct;131(2):206-18. doi: 10.1111/jnc.12814. Epub 2014 Jul 31. J Neurochem. 2014. PMID: 24995608
-
The allogeneic umbilical cord mesenchymal stem cells regulate the function of T helper 17 cells from patients with rheumatoid arthritis in an in vitro co-culture system.BMC Musculoskelet Disord. 2012 Dec 13;13:249. doi: 10.1186/1471-2474-13-249. BMC Musculoskelet Disord. 2012. PMID: 23237239 Free PMC article.
-
Suppressors of cytokine signalling in ankylosing spondylitis and their associations with disease severity, acute-phase reactants and serum cytokines.Clin Exp Rheumatol. 2016 Jan-Feb;34(1):100-5. Epub 2016 Jan 20. Clin Exp Rheumatol. 2016. PMID: 26812031
-
Activated and Naïve Allogenic Human Placental Mesenchymal Stromal Cells Exert an Immunomodulatory Effect on Hidradenitis Suppurativa Patient Peripheral Blood Mononuclear Cells.J Interferon Cytokine Res. 2024 Jul;44(7):291-299. doi: 10.1089/jir.2024.0035. Epub 2024 Apr 12. J Interferon Cytokine Res. 2024. PMID: 38607317
-
In vitro Immunomodulatory Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Peripheral Blood Cells from Warm Autoimmune Hemolytic Anemia Patients.Acta Haematol. 2022;145(1):63-71. doi: 10.1159/000506759. Epub 2021 Jul 20. Acta Haematol. 2022. PMID: 34284381 Clinical Trial.
References
-
- Pedersen SJ, Maksymowych WP. The pathogenesis of ankylosing spondylitis: an update. Curr Rheumatol Rep. 2019;21(10):58. 10.1007/s11926-019-0856-3 - PubMed
-
- Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34. 10.1136/ard-2022-223296 - PubMed
-
- Kroon FP, van der Burg LR, Ramiro S, Landewe RB, Buchbinder R, Falzon L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;2015(7):CD010952. 10.1002/14651858.CD010952.pub2 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

