Extent of lung fibrosis is of greater prognostic importance than HRCT pattern in patients with progressive pulmonary fibrosis: data from the ILD-PRO registry
- PMID: 40022059
- PMCID: PMC11871617
- DOI: 10.1186/s12931-025-03136-6
Extent of lung fibrosis is of greater prognostic importance than HRCT pattern in patients with progressive pulmonary fibrosis: data from the ILD-PRO registry
Abstract
Background: The prognostic value of patterns and quantitative measures of lung fibrosis on high-resolution computed tomography (HRCT) in patients identified as having progressive pulmonary fibrosis (PPF) has not been established. We investigated whether HRCT patterns and quantitative scores were associated with risk of progression in patients with PPF.
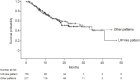
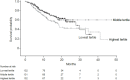
Methods: Patients enrolled in the ILD-PRO Registry had an interstitial lung disease (ILD) other than idiopathic pulmonary fibrosis, reticular abnormality and traction bronchiectasis, and met criteria for ILD progression. HRCT images taken between 24 months prior to enrollment and 90 days after enrollment were analyzed using a machine learning algorithm to derive quantitative scores. Associations were assessed between HRCT pattern (usual interstitial pneumonia [UIP]-like versus other patterns) and tertiles of quantitative scores and measures of disease severity at enrollment, and between these patterns/tertiles at enrollment and ILD progression (relative decline in forced vital capacity [FVC] % predicted ≥ 10%, lung transplant, or death) over a median follow-up of 17.3 months.
Results: Among 395 patients, 178 (45.1%) had a UIP-like pattern on HRCT. A UIP-like pattern did not associate with worse disease severity at enrollment or an increased risk of ILD progression (HR 1.01 [95% CI: 0.71, 1.44]). The highest quantitative lung fibrosis (QLF) score tertile (≥ 20.5%) was associated with worse disease severity. In unadjusted analyses, patients with QLF scores in the highest tertile had a significantly increased risk of ILD progression versus the middle tertile (HR [95% CI] 1.63 [1.07, 2.49] and a numerically increased risk versus the lowest tertile (HR 1.46 [0.97, 2.18]); however, after adjustment for sex, age, FVC % predicted and oxygen use at enrollment, there were no significant differences. There were no significant associations between tertiles of quantitative ILD score, quantitative ground glass score, or quantitative honeycomb cysts score and risk of ILD progression in unadjusted or adjusted analyses.
Conclusions: In a real-world cohort of patients with PPF, QLF score associated with subsequent risk of ILD progression, while HRCT pattern did not. The QLF score did not provide additional prognostic information beyond clinical variables.
Trial registration: ClinicalTrials.gov; No: NCT01915511; registered August 5, 2013; URL: www.
Clinicaltrials: gov .
Keywords: Disease progression; Interstitial lung disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Duke University Institutional Review Board (Pro00046131) and at every enrolling center (listed in the Acknowledgments). The protocol was approved by the relevant Institutional Review Boards and/or local Independent Ethics Committees prior to patient enrolment at every site. All patients provided written consent prior to entering the registry. Consent for publication: Not applicable. Competing interests: ACS, JMW, JLT, SMP and MLN are employed by the Duke Clinical Research Institute, which receives funding support from Boehringer Ingelheim Pharmaceuticals, Inc to coordinate the IPF-PRO/ILD-PRO Registry. ACS also reports consulting fees from United Therapeutics. JLT also reports grants from AstraZeneca and CareDx and has participated on advisory boards for Altavant, Avalyn, Natera, Sanofi, Theravance. SMP reports research funding paid to the Duke Clinical Research Institute from Bristol Myers Squibb and Genentech and has participated on advisory boards for Altavant and Bristol Myers Squibb. TPW is a site investigator for the IPF-PRO/ILD-PRO Registry. GHJK reports grants from Boehringer Ingelheim and Genentech; consulting fees from Voiant Clinical (formerly MedQIA); and holds patent UC-2015-0324982-A1. TBL was an employee of Boehringer Ingelheim Pharmaceuticals, Inc at the time that these analyses were performed. JG is the founder of MedQIA, now Voiant Clinical, which received funding support from Boehringer Ingelheim Pharmaceuticals, Inc, to analyze HRCT scans for this project; he also reports grants from Boehringer Ingelheim. The University of California Los Angeles has a patent for the machine learning algorithm used in this project.
Figures
References
-
- Selman M, Pardo A. From pulmonary fibrosis to progressive pulmonary fibrosis: a lethal pathobiological jump. Am J Physiol Lung Cell Mol Physiol. 2021;321:L600–7. - PubMed
-
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–27. - PubMed
-
- George PM, Spagnolo P, Kreuter M, Altinisik G, Bonifazi M, Martinez FJ, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med. 2020;8:925–34. - PubMed
-
- Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8:147–57. - PubMed

