Lassa virus protein-protein interactions as mediators of Lassa fever pathogenesis
- PMID: 40022100
- PMCID: PMC11869472
- DOI: 10.1186/s12985-025-02669-y
Lassa virus protein-protein interactions as mediators of Lassa fever pathogenesis
Abstract
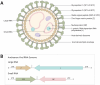
Viral hemorrhagic Lassa fever (LF), caused by Lassa virus (LASV), is a significant public health concern endemic in West Africa with high morbidity and mortality rates, limited treatment options, and potential for international spread. Despite advances in interrogating its epidemiology and clinical manifestations, the molecular mechanisms driving pathogenesis of LASV and other arenaviruses remain incompletely understood. This review synthesizes current knowledge regarding the role of LASV host-virus interactions in mediating the pathogenesis of LF, with emphasis on interactions between viral and host proteins. Through investigation of these critical protein-protein interactions, we identify potential therapeutic targets and discuss their implications for development of medical countermeasures including antiviral drugs. This review provides an update in recent literature of significant LASV host-virus interactions important in informing the development of targeted therapies and improving clinical outcomes for LF patients. Knowledge gaps are highlighted as opportunities for future research efforts that would advance the field of LASV and arenavirus pathogenesis.
Keywords: Arenavirus; Emerging RNA virus; Hemorrhagic fever virus; Host-virus interactions; Lassa fever pathogenesis; Lassa virus; Protein–protein interactions.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

