Prenatal inflammation exacerbates hyperoxia-induced neonatal brain injury
- PMID: 40022130
- PMCID: PMC11871844
- DOI: 10.1186/s12974-025-03389-4
Prenatal inflammation exacerbates hyperoxia-induced neonatal brain injury
Abstract
Background: Premature born infants are at high risk to develop white matter injury (WMI). Hyperoxia and perinatal inflammation are main risk factors for preterm birth and associated brain injury. To date the majority of experimental studies have focused on isolated insults. However, clinically, WMI injury is a multifactorial disorder caused by a variety of triggers. To establish a clinically relevant rodent model of WMI, we combined prenatal inflammation with postnatal hyperoxia to investigate individual, and additive or synergistic effects on inflammatory processes, myelination and grey matter development.
Methods: At embryonic day 20, pregnant Wistar rat dams received either a single intraperitoneal injection of 100 µg/ kg lipopolysaccharide (LPS) or sodium chloride. Offspring were either exposed to hyperoxia (80% O2) or normoxia (21% O2) from postnatal day 3 to 5. Animals were sacrificed immediately after hyperoxia or 6 days later, corresponding to term-equivalent age. White and grey matter development and neuroinflammatory responses were investigated at cellular and molecular levels applying immunohistochemistry, western blotting, real time PCR in brain tissues and multiplex protein expression analysis on serum samples.
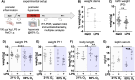
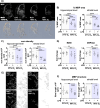
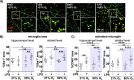
Results: Prenatal inflammation combined with postnatal hyperoxia resulted in reduced body weight and length in the offspring, accompanied by increased serum leptin levels at term equivalent age. The altered body parameters, like body weight, were associated with decreased brain volume, thinning of deep cortical layers and hypomyelination. As potential underlying mechanisms, we identified severe myelination deficits and an increased microglia activation associated with elevated inflammatory cytokine expression in brain tissues, while peripheral cytokine levels were reduced. Interestingly, effects on body size were mainly mediated by prenatal LPS, independent of hyperoxia, while oligodendrocyte degeneration was mainly induced by postnatal hyperoxia, independent of prenatal inflammation. However, for the majority of pathological changes, including brain size, myelination deficits, microglia activation and inflammatory cytokine expression, additive or synergistic effects were detected.
Conclusion: Prenatal inflammation combined with postnatal hyperoxia results in aggravated myelination deficits and inflammatory responses compared to single insults, making it an ideal model to improve our understanding of the complex pathophysiology underlying WMI and to evaluate urgently needed therapies.
Keywords: Cytokines; Microglia; Neuroinflammation; Oligodendrocytes; Postnatal hyperoxia; Prenatal inflammation; Preterm birth; White matter injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal experiments were performed in accordance with the Animal Research Reporting of in Vivo Experiments (ARRIVE) guidelines with governmental approval by the State Agency for Nature, Environment and Consumer Protection North Rhine-Westphalia. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Keller M, et al. Policy benchmarking report on neonatal health and social policies in 13 European countries. Acta Paediatr. 2010;99(11):1624–9. - PubMed
-
- Wood NS, et al. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343(6):378–84. - PubMed
-
- Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010;126(2):342–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

