The m6A demethylase FTO promotes C/EBPβ-LIP translation to perform oncogenic functions in breast cancer cells
- PMID: 40022434
- PMCID: PMC12103066
- DOI: 10.1111/febs.70033
The m6A demethylase FTO promotes C/EBPβ-LIP translation to perform oncogenic functions in breast cancer cells
Abstract
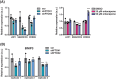
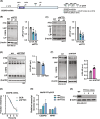
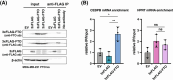
N6-methyladenosine (m6A) is a prevalent posttranscriptional mRNA modification involved in the regulation of transcript turnover, translation, and other aspects of RNA fate. The modification is mediated by multicomponent methyltransferase complexes (so-called writers) and is reversed through the action of the m6A-demethylases fat mass and obesity-associated (FTO) and alkB homolog 5 (ALKBH5) (so-called erasers). FTO promotes cell proliferation, colony formation and metastasis in models of triple-negative breast cancer (TNBC). However, little is known about genome-wide or specific downstream regulation by FTO. Here, we examined changes in the genome-wide transcriptome and translatome following FTO knockdown in TNBC cells. Unexpectedly, FTO knockdown had a limited effect on the translatome, while transcriptome analysis revealed that genes related to extracellular matrix (ECM) and epithelial-mesenchymal transition (EMT) are regulated through yet unidentified mechanisms. Differential translation of CEBPB mRNA into the C/EBPβ transcription factor isoform C/EBPβ-LIP is known to act in a pro-oncogenic manner in TNBC cells through regulation of EMT genes. Here we show that FTO is required for efficient C/EBPβ-LIP expression, suggesting that FTO has oncogenic functions through regulation of C/EBPβ-LIP.
Keywords: C/EBPβ; FTO; breast cancer; mRNA translation.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no competing interests.
Figures
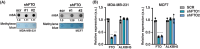






References
-
- Wei CM & Moss B (1977) Nucleotide sequences at the N6‐methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 16, 1672–1676. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

