A differentiated β-globin gene replacement strategy uses heterologous introns to restore physiological expression
- PMID: 40022449
- PMCID: PMC11997512
- DOI: 10.1016/j.ymthe.2025.02.036
A differentiated β-globin gene replacement strategy uses heterologous introns to restore physiological expression
Abstract
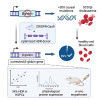
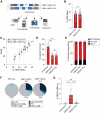
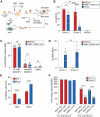
β-Hemoglobinopathies are common monogenic disorders. In sickle cell disease (SCD), a single mutation in the β-globin (HBB) gene results in dysfunctional hemoglobin protein, while in β-thalassemia, over 300 mutations distributed across the gene reduce β-globin levels and cause severe anemia. Genetic engineering replacing the whole HBB gene through homology-directed repair (HDR) is an ideal strategy to restore a benign genotype and rescue HBB expression for most genotypes. However, this is technically challenging because (1) the insert must not be homologous to the endogenous gene and (2) synonymous codon-optimized, intron-less sequences may not reconstitute adequate β-globin levels. Here, we developed an HBB gene replacement strategy using CRISPR-Cas9 that successfully addresses these challenges. We determined that a DNA donor containing a diverged HBB coding sequence and heterologous introns to avoid sequence homology provides proper physiological expression. We identified a DNA donor that uses truncated γ-globin introns, results in 34% HDR, and rescues β-globin expression in in vitro models of SCD and β-thalassemia in hematopoietic stem and progenitor cells (HSPCs). Furthermore, while HDR allele frequency dropped in vivo, it was maintained at ∼15%, demonstrating editing of long-term repopulating HSPCs. In summary, our HBB gene replacement strategy offers a differentiated approach by restoring naturally regulated adult hemoglobin expression.
Keywords: CRISPR-Cas9; HBB; HDR; gene editing; gene replacement; hematopoietic stem cells; hemoglobinopathies; sickle cell disease; β-thalassemia.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors are former employees of Graphite Bio, Inc. and may own stock/options in the company, and all work was done while authors were employed at Graphite Bio, Inc.. A patent application encompassing aspects of this work has been filed, with C.B., A.R.C., D.P.D., and B.W. included as inventors and Graphite Bio, Inc. as the applicant (PCT/US2022/024477, pending). M.H.P. serves on the scientific advisory board of Allogene Tx and is an advisor to Versant Ventures; he has equity in CRISPR Tx and serves on the board of directors of Kamau Therapeutics; and he is affiliated with Stanford University. J.L.G. is an advisor to Kamau Therapeutics. K.A.W. and M.H.P. are current employees and shareholders of Kamau Therapeutics. B.W. is affiliated with and has equity in Evercrisp Biosciences. T.L.G. and L.C. are affiliated with Scribe Therapeutics. C.L.E. is affiliated with the University of California, San Francisco. J.A.P.-B. is affiliated with Genentech. J.R.P. is affiliated with Novartis. W.M.M. and A.R.C. are affiliated with Enceladus Biosciences. K.K. is affiliated with Bio-Rad Laboratories. S.K. is affiliated with and has equity in CRISPR Tx. C.D.L. is affiliated with OmniAb. G.M.C. is affiliated with Caribou Biosciences. J.E.V. is affiliated with Alector. B.J.S. is affiliated with Senti Biosciences. B.J.Q. is affiliated with the University of California, San Diego. K.A.H. is affiliated with Amber Biosciences. J.L.G. is affiliated with Biogen.
Figures


References
-
- Kato G.J., Piel F.B., Reid C.D., Gaston M.H., Ohene-Frempong K., Krishnamurti L., Smith W.R., Panepinto J.A., Weatherall D.J., Costa F.F., Vichinsky E.P. Sickle cell disease. Nat. Rev. Dis. Primers. 2018;4 - PubMed
-
- Thein S.L. Pathophysiology of beta thalassemia--a guide to molecular therapies. Am. Soc. Hematol. Educ. Program. 2005:31–37. - PubMed
-
- Charache S., Terrin M.L., Moore R.D., Dover G.J., Barton F.B., Eckert S.V., McMahon R.P., Bonds D.R. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 1995;332:1317–1322. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

