Serotonergic Psychedelics Rapidly Modulate Evoked Glutamate Release in Cultured Cortical Neurons
- PMID: 40022486
- PMCID: PMC11871419
- DOI: 10.1111/jnc.70020
Serotonergic Psychedelics Rapidly Modulate Evoked Glutamate Release in Cultured Cortical Neurons
Abstract
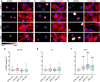
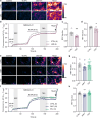
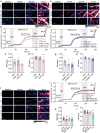
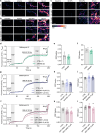
The serotonergic psychedelics psilocybin, LSD and DMT hold great promise for the development of new treatments for psychiatric conditions such as major depressive disorder, addiction and end-of-life anxiety. Previous studies in both animals and humans have confirmed the effects of these drugs on neuronal activity and plasticity. However, the understanding of the mechanisms of action of these substances is limited. Here we show rapid effects of psychedelics on presynaptic properties, using live cell imaging at the level of single synapses in primary rat cortical neurons. Using the genetically encoded reporter of synaptic vesicle fusion synaptopHluorin, we detected a reduced fraction of synaptic vesicles that fused in response to mild or strong electrical stimulation 3-30 min after application of serotonergic psychedelics. These effects were transient and no longer present 24 h after treatment. While DMT only reduced the total recycling pool, LSD and psilocin also reduced the size of the readily releasable vesicle pool. Imaging with the sensors for glutamate, iGluSnFR, and presynaptic calcium, synGCaMP6, showed that while psilocin and DMT increased evoked glutamate release, LSD and psilocin reduced evoked presynaptic calcium levels. Interestingly, psilocin also affected short-term plasticity leading to a depression of responses to paired stimuli. The rapid and drug-specific modulation of glutamatergic neurotransmission described in this study may contribute to distinct anxiolytic and antidepressant properties of serotonergic psychedelics.
Keywords: 5‐HT2A; fluorescent sensors; neurotransmitter release; presynaptic; short‐term plasticity; synaptic vesicles.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
Tomáš Páleníček declares to have shares in ‘Psyon s.r.o.’, has founded ‘PSYRES–Psychedelic Research Foundation’, and has shares in ‘Společnost pro podporu neurovědního výzkumu s.r.o.’ T.P furthermore reports consulting fees from GH Research and CB21‐Pharma outside the submitted work. T.P is involved in clinical trials of Compass Pathways with psilocybin, MAPS trial with MDMA and GH Research trial with 5‐MeO‐DMT outside the submitted work. The other authors have no relevant financial or non‐financial interests to disclose.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

