Buprenorphine blunts inflammatory response and wound progression after skin exposure to nitrogen mustard
- PMID: 40022640
- PMCID: PMC11871815
- DOI: 10.1111/wrr.70009
Buprenorphine blunts inflammatory response and wound progression after skin exposure to nitrogen mustard
Abstract
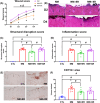
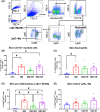
Opioid analgesics are often used to alleviate pain in rodent models of skin wound injury and repair. However, previous studies have demonstrated that opioids can alter the inflammatory response to injury and subsequent wound healing. The purpose of this study was to determine the impact of different formulations of buprenorphine on mouse behaviour, inflammatory response and wound progression following skin exposure to nitrogen mustard (NM). Administration of either short-acting or long-acting formulations of buprenorphine in conjunction with skin NM exposure resulted in body weight loss, reduced activity and behavioural changes. Both short-acting and long-acting formulations also dampened aspects of the inflammatory response to NM exposure, including reduced levels of the chemokines CCL3 and CXCL2 and reduced accumulation of pro-inflammatory macrophages. The diminished inflammatory response was associated with reduced skin injury assessed both externally and histologically. These results have important implications for the use of opioid analgesics in studies involving vesicant exposure as well as the potential for the use of opioids as a countermeasure after NM exposure.
Keywords: inflammation; mouse model; opioid analgesic; vesicant injury.
© 2025 The Author(s). Wound Repair and Regeneration published by Wiley Periodicals LLC on behalf of The Wound Healing Society.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

