Outer radial glia promotes white matter regeneration after neonatal brain injury
- PMID: 40023165
- PMCID: PMC11970391
- DOI: 10.1016/j.xcrm.2025.101986
Outer radial glia promotes white matter regeneration after neonatal brain injury
Abstract
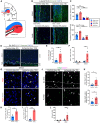
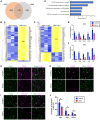
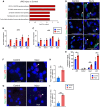
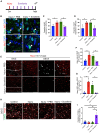
The developing gyrencephalic brain contains a large population of neural stem cells in the ventricular zone and outer subventricular zone (OSVZ), the latter populated by outer radial glia (oRG). The role of oRG during postnatal development is not well understood. We show that oRG cells increase proliferative capacity and contribute to oligodendrocyte precursor cell (OPC) production following brain injury in human infants and neonatal piglets, whose brains resemble the human brain in structure and development. RNA sequencing revealed oRG-specific transcriptional responses to injury in piglets and showed that the activating transcription factor 5 (ATF5) pathway positively regulates oRG proliferation. Intranasal activation of ATF5 using salubrinal enhanced OSVZ-derived oligodendrogenesis in the injured periventricular white matter and improved functional recovery. These results reveal a key role for postnatal oRG in brain injury recovery and identify ATF5 as a potential therapeutic target for treating white matter injury in infants.
Keywords: ATF5; MCL1; diffusion tensor imaging; gait behavior; neural stem cells; oligodendrocyte precursor cells; outer radial glia; outer subventricular zone; piglet brain; white matter regeneration.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

