Efficacy and Safety of 4-Month Rifapentine-Based Tuberculosis Treatments in Persons with Diabetes
- PMID: 40023788
- PMCID: PMC11878306
- DOI: 10.3201/eid3103.241634
Efficacy and Safety of 4-Month Rifapentine-Based Tuberculosis Treatments in Persons with Diabetes
Abstract
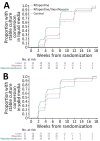
A previous study demonstrated noninferior efficacy of 4-month rifapentine/moxifloxacin regimen for tuberculosis (TB) treatment compared with the standard regimen. We explored results among study participants with diabetes. Among 2,516 randomized participants, 181 (7.2%) had diabetes. Of 166 participants with diabetes in the microbiologically eligible analysis group, 26.3% (15/57) had unfavorable outcomes in the control regimen, 13.8% (8/58) in the rifapentine/moxifloxacin regimen, and 29.4% (15/51) in the rifapentine regimen. The difference in proportion of unfavorable outcomes between the control and rifapentine/moxifloxacin arms in the microbiologically eligible analysis group was -12.5% (95% CI -27.0% to 1.9%); the difference between the control and rifapentine arms was 3.1% (95% CI -13.8% to 20.0%). Safety outcomes were similar in the rifapentine/moxifloxacin regimen and control arms. Among participants with TB and diabetes, the rifapentine/moxifloxacin arm had fewest unfavorable outcomes and was safe. Our findings indicate that the rifapentine/moxifloxacin regimen can be used in persons with TB and diabetes.
Keywords: United States; antimicrobial resistance; bacteria; diabetes; moxifloxacin; phase 3 clinical trial; respiratory infections; rifapentine; tuberculosis and other mycobacteria.
Figures

References
-
- World Health Organization. Global tuberculosis report 2023. 2023 Nov 7 [cited 2024 Aug 2]. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa...

