Evaluation of High-Dose Isoniazid in Multidrug-Resistant Tuberculosis Treatment
- PMID: 40023825
- PMCID: PMC11878312
- DOI: 10.3201/eid3103.241473
Evaluation of High-Dose Isoniazid in Multidrug-Resistant Tuberculosis Treatment
Abstract
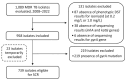
High-dose isoniazid is recommended to treat multidrug-resistant tuberculosis (MDR TB). Among 958 MDR TB isolates identified in France during 2008-2022, 93.1% exhibited high-level isoniazid resistance, and molecular testing showed limited diagnostic accuracy in predicting resistance. Clinicians should reconsider using high-dose isoniazid in MDR TB treatment because of suboptimal effect and toxicity concerns.
Keywords: MDR TB; SCR; antimicrobial resistance; bacteria; drug susceptibility testing; genotypic; hepatotoxicity; inhA; isoniazid; katG; mutation; phenotypic; respiratory infections; tuberculosis and other mycobacteria.
Figures
References
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment, 2022 update. Geneva: The Organization; 2022. - PubMed
-
- Gausi K, Ignatius EH, De Jager V, Upton C, Kim S, McKhann A, et al. High-dose isoniazid lacks EARLY bactericidal activity against isoniazid-resistant tuberculosis mediated by katG mutations: a randomized, phase 2 clinical trial. Am J Respir Crit Care Med. 2024;210:343–51. 10.1164/rccm.202311-2004OC - DOI - PMC - PubMed
-
- World Health Organization. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). Geneva: The Organization; 2021.
-
- World Health Organization. Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance, 2nd ed. Geneva: The Organization; 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources