Embryo-Induced Changes in the Protein Profile of Bovine Oviductal Extracellular Vesicles
- PMID: 40024377
- PMCID: PMC11994978
- DOI: 10.1016/j.mcpro.2025.100935
Embryo-Induced Changes in the Protein Profile of Bovine Oviductal Extracellular Vesicles
Abstract
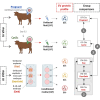
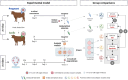
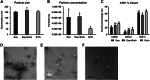
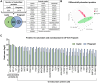
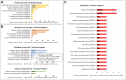
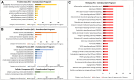
The study of early maternal-embryonic cross-talk remains one of the most challenging topics in reproductive biology. Understanding the physiological mechanisms involved in the interactions between the maternal reproductive tract and the developing embryo is essential for enhancing bovine reproductive efficiency. This complex communication starts within the oviduct, where the modulation of biological processes important for ensuring embryo quality is partially facilitated through extracellular vesicles (EVs). Utilizing a combination of in vivo and in vitro models this study had three main objectives: 1) to examine the protein cargo of EVs isolated from the oviductal fluid (OF) of cyclic and pregnant heifers to understand their role in maternal-embryonic communication in vivo; 2) to characterize the protein profile of EVs in conditioned medium (CM) resulting from the culture of oviductal explants alone (Exp) or in the presence of 8- to 16-cell stage embryos (Exp + Emb); and 3) to compare the protein cargo of EVs from Exp with EVs from cyclic heifers and EVs from Exp + Emb with EVs from pregnant heifers. Proteins were considered "identified" if detected in at least three out of five replicates and considered "exclusive" if detected in at least three out of five replicates within one group but absent in all samples of other groups. We identified 659 and 1476 proteins in the OF-EVs of cyclic and pregnant heifers, respectively. Among these, 644 proteins were identified in OF-EVs from both cyclic and pregnant heifers, and 40 proteins were exclusive to OF-EVs from the pregnant group. Within the 644 proteins identified in both groups, 31 were identified as differently abundant proteins (DAPs). In pregnant heifers, DAPs were mainly related to genome activation, DNA repair, embryonic cell differentiation, migration, and immune tolerance. In vitro, we identified 841 proteins in the CM-EVs from Exp alone, 613 from Exp + Emb, and 111 in the CM-EVs from Emb alone. In the qualitative analysis between the three in vitro groups, 81 proteins were identified in all groups, 452 were common to Exp and Exp + Emb, 17 were common to Exp and Emb, 5 were common to Exp + Emb and Emb, 4 were unique to Exp, 6 were unique to Exp + Emb, and none were unique to Emb. Proteins identified when there is an interaction between the oviduct and the embryo in vitro, corresponding to the Exp + Emb group, were associated with immune tolerance, structural activity, binding, and cytoskeletal regulation. In vivo and in vitro EVs exhibit distinct qualitative and quantitative protein contents, both when comparing EVs produced in the absence of an embryo (Cyclic and Exp) and those that have undergone embryo-oviduct interaction (Pregnant and Exp + Emb). The observed changes in the protein cargo of EVs due to maternal-embryonic communication in vivo and in vitro suggest that the interaction between the embryo and the maternal milieu initiates within the oviduct and is potentially facilitated by EVs and their protein contents.
Keywords: bovine; embryo-maternal interaction; exracellular vesicles; in vitro; in vivo; oviduct; pregnancy.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Rizos D., Ward F., Duffy P., Boland M.P., Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002;61:234–248. - PubMed
-
- Lonergan P., Rizos D., Gutierrez-Adán A., Moreira P.M., Pintado B., de la Fuente J., et al. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol. Reprod. 2003;69:1424–1431. - PubMed
-
- Khurana N.K., Niemann H. Energy metabolism in preimplantation bovine embryos derived in vitro or in Vivo1. Biol. Reprod. 2000;62:847–856. - PubMed
-
- Rizos D., Clemente M., Bermejo-Alvarez P., De La Fuente J., Lonergan P., Gutiérrez-Adán A. Consequences of in vitro culture conditions on embryo development and quality. Reprod. Domest. Anim. 2008;43:44–50. - PubMed
-
- Rizos D., Fair T., Papadopoulos S., Boland M.P., Lonergan P. Developmental, qualitative, and ultrastructural differences between ovine and bovine embryos produced in vivo or in vitro. Mol. Reprod. Dev. 2002;62:320–327. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

