Epicutaneous immunotherapy for food allergy: A systematic review and meta-analysis
- PMID: 40024882
- PMCID: PMC11872371
- DOI: 10.1002/clt2.70045
Epicutaneous immunotherapy for food allergy: A systematic review and meta-analysis
Abstract
Background: Food allergies pose a global healthcare challenge, underscoring the need for effective interventions. This study evaluated the efficacy and safety of epicutaneous immunotherapy (EPIT) for food allergen desensitisation.
Methods: We conducted a systematic review of randomised controlled trials by searching Ovid EMBASE, PubMed and Scopus in April 2024. Using a random-effects meta-analysis, we evaluated the clinical effectiveness and harms of EPIT, reporting results as risk ratios with 95% confidence intervals (CI).
Results: After screening 460 abstracts and 35 full reports, 11 were included: nine on peanuts and two on cow's milk (CM). Peanut EPIT had a 51.2% treatment response versus 22.4% for placebo (RR 2.16, CI 1.49-3.12; four studies; moderate certainty). The RR for milk EPIT response rate was 1.78 (CI 1.06-3.00; one study). Five peanut studies (1396 patients) reported EPIT-related adverse events (RR 1.39, CI 0.94-2.05; low certainty).
Conclusions: EPIT offers a moderate treatment response with a favourable safety profile and significant improvements in quality of life. Current knowledge of EPIT remains limited, with evidence confined to peanut and CM allergies. There is a lack of research on sustained unresponsiveness achieved through food EPIT.
Keywords: EPIT; allergen immunotherapy; cow's milk; epicutaneous immunotherapy; peanuts.
© 2025 The Author(s). Clinical and Translational Allergy published by John Wiley & Sons Ltd on behalf of European Academy of Allergy and Clinical Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


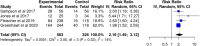
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

