Oral vs. Outpatient Parenteral Antimicrobial Treatment for Infective Endocarditis: Study Protocol for the Spanish OraPAT-IE GAMES Trial
- PMID: 40024946
- PMCID: PMC11933631
- DOI: 10.1007/s40121-025-01110-9
Oral vs. Outpatient Parenteral Antimicrobial Treatment for Infective Endocarditis: Study Protocol for the Spanish OraPAT-IE GAMES Trial
Abstract
Introduction: The POET trial demonstrated that moving from intravenous to oral antibiotics in stable patients with left-sided infective endocarditis (IE) was noninferior to fully parenteral treatment. However, it did not compare outpatient strategies.
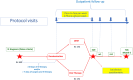
Methods: The OraPAT-IE GAMES trial is a noninferiority, multicenter, randomized, open-label study aimed to compare partial oral versus outpatient parenteral antibiotic therapy (OPAT) for consolidation of antibiotic treatment in left-sided IE. A total of 342 stable patients with IE caused by selected micro-organisms will eventually be included. After a minimum of 10 days of parenteral treatment, stable patients are randomized to oral therapy or OPAT. The primary end-point is a composite of all-cause mortality, unplanned cardiac surgery, relapse of positive blood cultures and/or unplanned hospital admission. Patients are followed-up for 6 months after completing antibiotic therapy.
Planned outcome: This trial seeks to demonstrate the equivalent efficacy of the two outpatient strategies currently available for stable patients with IE in the consolidation phase of antibiotic treatment.
Conclusion: In a global context of limited healthcare resources and a sustained increase in elderly and frail patients, it is of great importance to demonstrate the effectiveness and safety of outpatient management strategies that could reduce the duration of conventional hospitalizations with their potential complications and inherent costs.
Trial registration: EudraCT: 2020-001024-34.
Clinicaltrials: gov identifier: NCT05398679.
Keywords: Infective endocarditis; Mortality; OPAT; Oral antibiotic therapy; Oral step-down antibiotic treatment; Outpatient parenteral antibiotic treatment; Partial oral treatment; Randomized controlled trial; Reinfections; Relapses.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical Approval: The trial will be conducted according to the principles of the last Declaration of Helsinki (accorded by the 64th World Medical Association General Assembly in 2013), the Good Clinical Practice (GCP) and current legislation. The investigator is responsible to guarantee that clinical trial is realized following the directives established by the International Conference on Harmonization about GCP and local legislation. The study was authorized by the Spanish Medicines and Healthcare Products Regulatory Agency (Agencia Española de Medicamentos y Productos Sanitarios / AEMPS) and the Ethic Committee. The trial protocol received the AEMPS approval in December 15th 2021 and the research ethics committee approval in March 8th 2022. A substantial amendment received AEMPs approval and research ethics committee approval in January 25th and February 14th 2023, respectively. The principal investigator or collaborator at each site will provide the Information sheet to the patients and they will explain the study, objectives and clarify any doubt. They will obtain written informed consent from all patients, or their legal representatives (LRs) if they lack capacity, before enrolment. Patients (or their LRs) are free to withdraw from the trial at any time and this will be explicitly stated on the patient’s information sheets. Patient personal and clinical information will be managed according to European Regulation 2016/679 and Spanish legislation. Patient’s data will be anonymized, identifying every patient by a code. Only the study doctor and collaborators have access to clinical history. Consequently, the patient’s identity will not be revealed to any other person, except in cases of medical emergency or if required to do so by law. Access to patient information will be restricted to the study doctor and collaborators, the health authorities (AEMPS), the Clinical Research Ethics Committee, and personnel authorized by the sponsor when they need to check the data and procedures used in the study, but always maintaining the confidentiality of the said information in accordance with current legislation. This protocol has been formulated following the Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT) statement. This publication follows the Consolidated Standards of Reporting Trials (CONSORT) statement. Conflict of Interest: Juan Ambrosioni is an Editorial Board member of Infectious Diseases and Therapy. Juan Ambrosioni was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Jose M. Miro is a member of the Reial Academia de Medicina de Catalunya (RAMC), Barcelona, Spain. All named authors declare that they have no competing interests.
Figures
References
-
- Baddour LM, Wilson WR, Bayer AS, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–86. 10.1161/CIR.0000000000000296. - DOI - PubMed
-
- Forestier E, Roubaud-Baudron C, Fraisse T, AEPEI and the GInGer Elderl-IE Study Group, et al. Comprehensive geriatric assessment in older patients suffering from infective endocarditis. A prospective multicentric cohort study. Clin Microbiol Infect. 2019;25(10):1246–52. 10.1016/j.cmi.2019.04.021. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical