CD47 is required for mesenchymal progenitor proliferation and fracture repair
- PMID: 40025005
- PMCID: PMC11873311
- DOI: 10.1038/s41413-025-00409-0
CD47 is required for mesenchymal progenitor proliferation and fracture repair
Abstract
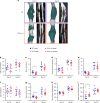
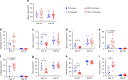
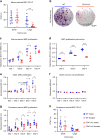
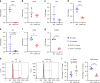
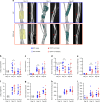
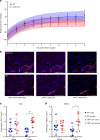
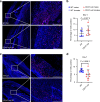
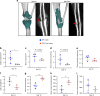
CD47 is a ubiquitous and pleiotropic cell-surface receptor. Disrupting CD47 enhances injury repair in various tissues but the role of CD47 has not been studied in bone injuries. In a murine closed-fracture model, CD47-null mice showed decreased callus bone formation as assessed by microcomputed tomography 10 days post-fracture and increased fibrous volume as determined by histology. To understand the cellular basis for this phenotype, mesenchymal progenitors (MSC) were harvested from bone marrow. CD47-null MSC showed decreased large fibroblast colony formation (CFU-F), significantly less proliferation, and fewer cells in S-phase, although osteoblast differentiation was unaffected. However, consistent with prior research, CD47-null endothelial cells showed increased proliferation relative to WT cells. Similarly, in a murine ischemic fracture model, CD47-null mice showed reduced fracture callus size due to a reduction in bone relative to WT 15 days-post fracture. Consistent with our in vitro results, in vivo EdU labeling showed decreased cell proliferation in the callus of CD47-null mice, while staining for CD31 and endomucin demonstrated increased endothelial cell density. Finally, WT mice with ischemic fracture that were administered a CD47 morpholino, which blocks CD47 protein production, showed a callus phenotype similar to that of ischemic fractures in CD47-null mice, suggesting the phenotype was not due to developmental changes in the knockout mice. Thus, inhibition of CD47 during bone healing reduces both non-ischemic and ischemic fracture healing, in part, by decreasing MSC proliferation. Furthermore, the increase in endothelial cell proliferation and early blood vessel density caused by CD47 disruption is not sufficient to overcome MSC dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Update of
-
CD47 is Required for Mesenchymal Progenitor Proliferation and Fracture Repair.bioRxiv [Preprint]. 2024 Mar 6:2024.03.06.583756. doi: 10.1101/2024.03.06.583756. bioRxiv. 2024. Update in: Bone Res. 2025 Mar 03;13(1):29. doi: 10.1038/s41413-025-00409-0. PMID: 38496546 Free PMC article. Updated. Preprint.
-
CD47 is Required for Mesenchymal Progenitor Proliferation and Fracture Repair.Res Sq [Preprint]. 2024 Mar 19:rs.3.rs-4022423. doi: 10.21203/rs.3.rs-4022423/v1. Res Sq. 2024. Update in: Bone Res. 2025 Mar 03;13(1):29. doi: 10.1038/s41413-025-00409-0. PMID: 38562718 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- F30AR071201/U.S. Department of Health & Human Services | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- R01 AR066028/AR/NIAMS NIH HHS/United States
- T32TR004371/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- T32 TR004371/TR/NCATS NIH HHS/United States
- F30 AR071201/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

