Molecular glues of the regulatory ChREBP/14-3-3 complex protect beta cells from glucolipotoxicity
- PMID: 40025013
- PMCID: PMC11873037
- DOI: 10.1038/s41467-025-57241-7
Molecular glues of the regulatory ChREBP/14-3-3 complex protect beta cells from glucolipotoxicity
Abstract
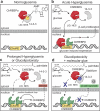
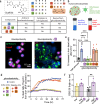
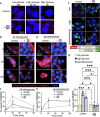
The Carbohydrate Response Element Binding Protein (ChREBP) is a glucose-responsive transcription factor (TF) with two major splice isoforms (α and β). In chronic hyperglycemia and glucolipotoxicity, ChREBPα-mediated ChREBPβ expression surges, leading to insulin-secreting β-cell dedifferentiation and death. 14-3-3 binding to ChREBPα results in cytoplasmic retention and suppression of transcriptional activity. Thus, small molecule-mediated stabilization of this protein-protein interaction (PPI) may be of therapeutic value. Here, we show that structure-based optimizations of a 'molecular glue' compound led to potent ChREBPα/14-3-3 PPI stabilizers with cellular activity. In primary human β-cells, the most active compound retained ChREBPα in the cytoplasm, and efficiently protected β-cells from glucolipotoxicity while maintaining β-cell identity. This study may thus not only provide the basis for the development of a unique class of compounds for the treatment of Type 2 Diabetes but also showcases an alternative 'molecular glue' approach for achieving small molecule control of notoriously difficult to target TFs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: US provisional patent application No. 63/523,289—Pennings M.A.M., Katz L.S. Ottmann C., Scott D.K., Visser E.J., Plitzko K.F., Kaiser M., Cossar P.J., Brunsveld L. Small-molecule stabilizers of the ChREBP–14-3-3 interaction with cellular activity. United States Patent and Trademark Office, June 2023. The authors declare the following competing financial interest(s): L.B. and C.O. are founders of Ambagon Therapeutics. L.B. is a member of Ambagon’s scientific advisory board, C.O. is employee of Ambagon. All other Authors declare no competing interests.
Figures




Update of
-
Molecular glues of the regulatory ChREBP/14-3-3 complex protect beta cells from glucolipotoxicity.bioRxiv [Preprint]. 2024 Nov 17:2024.02.16.580675. doi: 10.1101/2024.02.16.580675. bioRxiv. 2024. Update in: Nat Commun. 2025 Mar 02;16(1):2110. doi: 10.1038/s41467-025-57241-7. PMID: 38405965 Free PMC article. Updated. Preprint.
References
-
- Ogurtsova, K. et al. IDF diabetes atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pr.183, 109118 (2022). - PubMed
-
- Tancredi, M. et al. Excess mortality among persons with type 2 diabetes. N. Engl. J. Med.373, 1720–1732 (2015). - PubMed
-
- Chatterjee, S., Khunti, K. & Davies, M. J. Type 2 diabetes. Lancet389, 2239–2251 (2017). - PubMed
-
- Schellenberg, E. S., Dryden, D. M., Vandermeer, B., Ha, C. & Korownyk, C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med.159, 543–551 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

