The relationship between cortical synaptic terminal density marker SV2A and glutamate early in the course of schizophrenia: a multimodal PET and MRS imaging study
- PMID: 40025026
- PMCID: PMC11873237
- DOI: 10.1038/s41398-025-03269-8
The relationship between cortical synaptic terminal density marker SV2A and glutamate early in the course of schizophrenia: a multimodal PET and MRS imaging study
Abstract
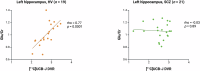
Loss of glutamatergic terminals is hypothesised to contribute to excitation-inhibition imbalance in schizophrenia, supported by evidence that the normal positive association between glutamate concentrations and synaptic terminal density is not found in patients with chronic schizophrenia. However, it is unknown whether the relationship between synaptic terminal density and glutamate levels is altered early in the course of illness. To address this, we investigated [11C]UCB-J distribution volume ratio (DVR) and glutamatergic markers in healthy volunteers (HV) and in antipsychotic-naïve/free patients with schizophrenia (SCZ) recruited from first-episode psychosis services. Forty volunteers (HV n = 19, SCZ n = 21) underwent [11C]UCB-J positron emission tomography and proton magnetic resonance spectroscopy (1H-MRS) imaging in the anterior cingulate cortex (ACC) and left hippocampus to index [11C]UCB-J DVR and creatine-scaled glutamate (Glu/Cr) and glutamate in combination with glutamine (Glx/Cr). In the HV but not SCZ group, [11C]UCB-J DVR was significantly positively associated with Glu/Cr (Spearman's rho = 0.55, p = 0.02) and Glx/Cr (Spearman's rho = 0.73, p = 0.0004) in the ACC, and with Glu/Cr in the left hippocampus (Spearman's rho = 0.77, p = 0.0001). DVR was significantly lower in the ACC in the SCZ group compared to the HV group (Kolmogorov-Smirnov Z = 1.44, p = 0.03). Together, these findings indicate that the normal relationship between levels of a synaptic terminal density marker and levels of glutamate is disrupted early in the course of schizophrenia. This is consistent with the hypothesis that there is loss of glutamatergic terminals at illness onset.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: SP has received honoraria as a consultant or speaker from Janssen, Recordati, Sunovion, Lundbeck, Rovi and Otsuka and a research grant from Recordati, all outside the submitted work. EAR and RNG are employees of Invicro LLC. AM was an employee of Invicro LLC during the completion of much of this work. TRM has received honoraria for speaking and chairing from Lundbeck, Janssen, and Astellas and received honoraria to participate in advisory boards organized by Angelini Pharmaceuticals. In the last 3 years, SJ has given nonpromotional educational talks for Lundbeck, Janssen, and Sunovion. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Angellini, Autifony, Biogen, Boehringer Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Recordati, Roche, and Viatris/Mylan. ODH has a patent for the use of dopaminergic imaging. The other authors report no biomedical financial interests or potential conflicts of interest.
Figures

References
-
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–77. - PubMed
-
- McGuire P, Howes OD, Stone J, Fusar-Poli P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci. 2008;29:91–8. - PubMed
-
- Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399:473–86. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

