HCN4 channels sense temperature and determine heart rate responses to heat
- PMID: 40025061
- PMCID: PMC11873294
- DOI: 10.1038/s41467-025-57358-9
HCN4 channels sense temperature and determine heart rate responses to heat
Abstract
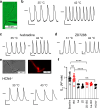
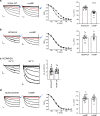
The hyperpolarization-activated cyclic nucleotide-gated ion channel 4 (HCN4) current increases due to cAMP binding and is well-recognized to contribute to adrenergically driven heart rate acceleration. HCN4 current also increases with heat by an unknown mechanism(s). We use thermodynamical and homology computational modeling, site-directed mutagenesis, and mouse models to identify a concise motif on the S4-S5 linker of HCN4 channels (M407/Y409) that determines HCN4 current (If) responses to heat. This motif is required for heat-triggered rate acceleration in cardiac pacemaker cells, isolated hearts and in vivo. Surprisingly, a loss of function M407/Y409 motif mutation prevented not only normal heat but also cAMP responses, suggesting that the heat-sensing machinery within the S4-S5 linker is essential for operating the cAMP allosteric pathway and is central to HCN4 gating modulation. The M407/Y409 motif is conserved across all HCN family members suggesting that HCN channels participate broadly in coupling heat to changes in cell membrane excitability.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
HCN channels sense temperature and determine heart rate responses to heat.bioRxiv [Preprint]. 2023 Sep 3:2023.09.02.556046. doi: 10.1101/2023.09.02.556046. bioRxiv. 2023. Update in: Nat Commun. 2025 Mar 01;16(1):2102. doi: 10.1038/s41467-025-57358-9. PMID: 37693513 Free PMC article. Updated. Preprint.
References
-
- Kirschen, G. W., Singer, D. D., Thode, H. C. Jr. & Singer, A. J. Relationship between body temperature and heart rate in adults and children: a local and national study. Am. J. Emerg. Med.38, 929–933 (2020). - PubMed
-
- Biel, M., Wahl-Schott, C., Michalakis, S. & Zong, X. Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev.89, 847–885 (2009). - PubMed
-
- DiFrancesco, D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol.55, 455–472 (1993). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

