Marine sulphate captures a Paleozoic transition to a modern terrestrial weathering environment
- PMID: 40025066
- PMCID: PMC11873193
- DOI: 10.1038/s41467-025-57282-y
Marine sulphate captures a Paleozoic transition to a modern terrestrial weathering environment
Abstract
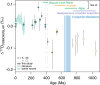
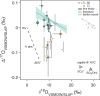
The triple oxygen isotope composition of sulphate minerals has been used to constrain the evolution of Earth's surface environment (e.g., pO2, pCO2 and gross primary productivity) throughout the Proterozoic Eon. This approach presumes the incorporation of atmospheric O2 atoms into riverine sulphate via the oxidative weathering of pyrite. However, this is not borne out in recent geological or modern sulphate records, where an atmospheric signal is imperceptible and where terrestrial pyrite weathering occurs predominantly in bedrock fractures that are physically more removed from atmospheric O2. To better define the transition from a Proterozoic to a modern-like weathering regime, here we present new measurements from twelve marine evaporite basins spanning the Phanerozoic. These data display a step-like transition in the triple oxygen isotope composition of evaporite sulphate during the mid-Paleozoic (420 to 387.7 million years ago). We propose that the evolution of early root systems deepened the locus of pyrite oxidation and reduced the incorporation of O2 into sulphate. Further, the early Devonian proliferation of land plants increased terrestrial organic carbon burial, releasing free oxygen that fueled increased redox recycling of soil-bound iron and resulted in the final rise in pO2 to modern-like levels.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Bergman, N. M., Lenton, T. M. & Watson, A. J. COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci.304, 397–437 (2004).
-
- Berner, R. A., Beerling, D. J., Dudley, R., Robinson, J. M. & Wildman, R. A. Jr Phanerozoic atmospheric oxygen. Annu. Rev. Earth Planet. Sci.31, 105–134 (2003).
-
- Scott, C. et al. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature452, 456–459 (2008). - PubMed

