A covalent chemical probe for Chikungunya nsP2 cysteine protease with antialphaviral activity and proteome-wide selectivity
- PMID: 40025188
- PMCID: PMC11873239
- DOI: 10.1038/s41598-025-91673-x
A covalent chemical probe for Chikungunya nsP2 cysteine protease with antialphaviral activity and proteome-wide selectivity
Abstract
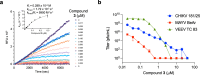
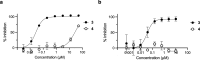
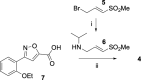
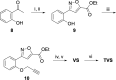
Chikungunya is a mosquito-borne viral disease that causes fever and severe joint pain for which there is no direct acting drug treatments. Vinyl sulfone SGC-NSP2PRO-1 (3) was identified as a potent inhibitor of the nsP2 cysteine protease (nsP2pro) that reduced viral titer against infectious isolates of Chikungunya and other alphaviruses. The covalent warhead in 3 captured the active site C478 and inactivated nsP2pro with a kinact/Ki ratio of 5950 M-1 s-1. The vinyl sulfone 3 was inactive across a panel of 23 other cysteine proteases and demonstrated remarkable proteome-wide selectivity by two chemoproteomic methods. A negative control analog SGC-NSP2PRO-1N (4) retained the isoxazole core and covalent warhead but demonstrated > 100-fold decrease in enzyme inhibition. Both 3 and 4 were stable across a wide range of pH in solution and upon prolonged storage as solids. Vinyl sulfone 3 and its negative control 4 will find utility as high-quality chemical probes to study the role of the nsP2pro in cellular studies of alphaviral replication and virulence.
Keywords: Alphavirus; Antiviral; Chemical probe; Covalent inhibitor; Cysteine protease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Update of
-
A Covalent Chemical Probe for Chikungunya nsP2 Cysteine Protease with Antialphaviral Activity and Proteome-wide Selectivity.Res Sq [Preprint]. 2024 Nov 12:rs.3.rs-5363451. doi: 10.21203/rs.3.rs-5363451/v1. Res Sq. 2024. Update in: Sci Rep. 2025 Mar 01;15(1):7264. doi: 10.1038/s41598-025-91673-x. PMID: 39606462 Free PMC article. Updated. Preprint.
References
-
- Suhrbier, A., Jaffar-Bandjee, M. C. & Gasque, P. Arthritogenic alphaviruses–an overview. Nat. Rev. Rheumatol.8, 420–429. 10.1038/nrrheum.2012.64 (2012). - PubMed
-
- Morens, D. M., Folkers, G. K. & Fauci, A. S. Eastern equine encephalitis virus - another emergent arbovirus in the united states. N. Engl. J. Med.381, 1989–1992. 10.1056/NEJMp1914328 (2019). - PubMed
-
- Massachusetts Department of Public Health. Massachusetts arbovirus updatehttps://www.mass.gov/info-details/massachusetts-arbovirus-update (2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

