The role of oxidative stress-mediated fibro-adipogenic progenitor senescence in skeletal muscle regeneration and repair
- PMID: 40025535
- PMCID: PMC11872320
- DOI: 10.1186/s13287-025-04242-4
The role of oxidative stress-mediated fibro-adipogenic progenitor senescence in skeletal muscle regeneration and repair
Abstract
Background: Stem cells play a pivotal role in tissue regeneration and repair. Skeletal muscle comprises two main stem cells: muscle stem cells (MuSCs) and fibro-adipogenic progenitors (FAPs). FAPs are essential for maintaining the regenerative milieu of muscle tissue and modulating the activation of muscle satellite cells. However, during acute skeletal muscle injury, the alterations and mechanisms of action of FAPs remain unclear.
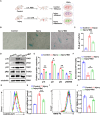
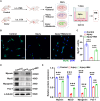
Methods: we employed the GEO database for bioinformatics analysis of skeletal muscle injury. A skeletal muscle injury model was established through cardiotoxin (CTX, 10µM, 50µL) injection into the tibialis anterior (TA) of C57BL/6 mice. Three days post-injury, we extracted the TA, isolated FAPs (CD31-CD45-PDGFRα+Sca-1+), and assessed the senescence phenotype through SA-β-Gal staining and Western blot. Additionally, we established a co-culture system to evaluate the capacity of FAPs to facilitate MuSCs differentiation. Finally, we alleviated the senescent of FAPs through in vitro (100 µM melatonin, 5 days) and in vivo (20 mg/kg/day melatonin, 15 days) administration experiments, confirming melatonin's pivotal role in the regeneration and repair processes of skeletal muscle.
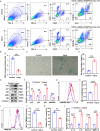
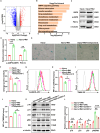
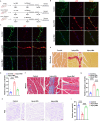
Results: In single-cell RNA sequencing analysis, we discovered the upregulation of senescence-related pathways in FAPs following injury. Immunofluorescence staining revealed the co-localization of FAPs and senescent markers in injured muscles. We established the CTX injury model and observed a reduction in the number of FAPs post-injury, accompanied by the manifestation of a senescent phenotype. Melatonin treatment was found to attenuate the injury-induced senescence of FAPs. Further co-culture experiments revealed that melatonin facilitated the restoration of FAPs' capacity to promote myoblast differentiation. Through GO and KEGG analysis, we found that the administration of melatonin led to the upregulation of AMPK pathway in FAPs, a pathway associated with antioxidant stress response. Finally, drug administration experiments corroborated that melatonin enhances skeletal muscle regeneration and repair by alleviating FAP senescence in vivo.
Conclusion: In this study, we first found FAPs underwent senescence and redox homeostasis imbalance after injury. Next, we utilized melatonin to enhance FAPs regenerative and repair capabilities by activating AMPK signaling pathway. Taken together, this work provides a novel theoretical foundation for treating skeletal muscle injury.
Keywords: FAPs; Melatonin; Senescence; Skeletal muscle injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were performed in accordance with approved protocols by the Institutional Animal Care and Use Committee (IACUC) of Shenzhen LingFu TopBiotech. Co., LTD. and were reported in line with the ARRIVE guidelines 2.0. (1) Title of the approved project: The Role of Oxidative Stress-Mediated Fibro-Adipogenic Progenitors Senescence in Skeletal Muscle Regeneration and Repair; (2) Name of the institutional approval committee: IACUC of Shenzhen LingFu TopBiotech. Co., LTD.; (3) Approval number: TOPGM-IACUC-2023-0200; (4) Date of approval: November 11th, 2023. Consent for publication: All authors have confirmed their consent for publication. Conflict of interest: The authors assert that they do not have any conflicts of interest.
Figures

Similar articles
-
Single-cell RNA-seq reveals novel interaction between muscle satellite cells and fibro-adipogenic progenitors mediated with FGF7 signalling.J Cachexia Sarcopenia Muscle. 2024 Aug;15(4):1388-1403. doi: 10.1002/jcsm.13484. Epub 2024 May 16. J Cachexia Sarcopenia Muscle. 2024. PMID: 38751367 Free PMC article.
-
Head muscle fibro-adipogenic progenitors account for the tilted regeneration towards fibrosis.Biochem Biophys Res Commun. 2022 Jan 22;589:131-138. doi: 10.1016/j.bbrc.2021.12.009. Epub 2021 Dec 3. Biochem Biophys Res Commun. 2022. PMID: 34915407
-
The Role of Matrix Metalloproteinase-13 (MMP13) in TGFβ/BMP Pathway Regulation of Fibro-Adipogenic Progenitor (FAP) Differentiation.Cell Physiol Biochem. 2022 Dec 20;56(6):730-743. doi: 10.33594/000000596. Cell Physiol Biochem. 2022. PMID: 36537139
-
Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease.FEBS J. 2022 Nov;289(21):6484-6517. doi: 10.1111/febs.16080. Epub 2021 Jul 6. FEBS J. 2022. PMID: 34143565 Review.
-
Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases.Open Biol. 2021 Dec;11(12):210110. doi: 10.1098/rsob.210110. Epub 2021 Dec 8. Open Biol. 2021. PMID: 34875199 Free PMC article. Review.
References
-
- Cossu G, Birchall M, Brown T, De Coppi P, Culme-Seymour E, Gibbon S, Hitchcock J, Mason C, Montgomery J, Morris S, et al. Commission: stem cells and regenerative medicine. Lancet. 2018;391(10123):883–910. - PubMed
-
- Yoshimoto Y, Oishi Y. Mechanisms of skeletal muscle-tendon development and regeneration/healing as potential therapeutic targets. Pharmacol Ther. 2023;243:108357. - PubMed
-
- Chazaud B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 2020;41(6):481–92. - PubMed
-
- Espino-Gonzalez E, Dalbram E, Mounier R, Gondin J, Farup J, Jessen N, Treebak JT. Impaired skeletal muscle regeneration in diabetes:from cellular and molecular mechanisms to novel treatments. Cell Metabol. 2024;36(6):1204–36. - PubMed
-
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–43. - PubMed
MeSH terms
Substances
Grants and funding
- 32170799/the National Natural Science Foundation of China
- 2023B1515020016/Guangdong Basic and Applied Basic Research Foundation
- 393011/Research Start-up Fund of the Seventh Affiliated Hospital,Sun Yat-sen University
- 23ykbj003/Fundamental Research Funds for the Central Universities, Sun Yat-sen University
- 00201314003/Shenzhen Medical Research Fund
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

