Diagnosis of hereditary transthyretin amyloidosis in patients with suspected chronic inflammatory demyelinating polyneuropathy unresponsive to intravenous immunoglobulins: results of a retrospective study
- PMID: 40025610
- PMCID: PMC11871584
- DOI: 10.1186/s13023-025-03589-4
Diagnosis of hereditary transthyretin amyloidosis in patients with suspected chronic inflammatory demyelinating polyneuropathy unresponsive to intravenous immunoglobulins: results of a retrospective study
Abstract
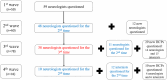
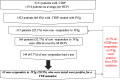
Background and aims: Hereditary transthyretin amyloidosis (ATTRv) should be considered in patients diagnosed with intravenous immunoglobulin (IVIg)-resistant chronic inflammatory demyelinating polyradiculoneuropathy (IVIg-NR CIDP). In this 1-year long, retrospective, multicentric study, an online questionnaire was sent to 1100 French healthcare professionals (HCPs) investigating: (i) how many IVIg-NR CIDP patients they followed; (ii) how many IVIg-NR CIDP patients had undergone TTR gene analysis; and (iii) how many IVIg-NR CIDP patients were eventually diagnosed with ATTRv. The questionnaire was sent every 3 months for 1 year and contained information on ATTRv clinical manifestations and diagnosis.
Results: One-hundred and ten (10%) HCPs responded. A total of 2131 patients with CIDP were identified, including 315 (22.1%) with IVIg-NR CIDP. TTR gene analysis was performed in 144 patients and was positive in 43 cases (29.9%).
Conclusions: This study demonstrates that ATTRv should be investigated systematically in patients diagnosed with IVIg-NR CIDP. HCP-directed information campaigns are useful for modifying diagnostic practices.
Keywords: Chronic inflammatory demyelinating polyradiculoneuropathy; Hereditary transthyretin amyloidosis; Intravenous immunoglobulins.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: DA has received consulting fees from Alnylam and Astra Zeneca. DA has received support for attending meetings and travel from Alnylam. SA has received consulting fees from Alnylam, Pfizer and Astra Zeneca. SA has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Alnylam, Pfizer and Astra Zeneca. SA has received support for attending meetings from Alnylam and Pfizer. JBC has received support for attending meetings from Alnylam, CSL Behring, LFB and Pfizer. JBC has participated on a Data Safety Monitoring Board or Advisory Board for Alnylam. PC has received consulting fees from Alnylam. PC has received honoraria for presentations from Alnylam. LM has received consulting fees from Alnylam, Pfizer and LFB. LM received support for attending meetings and/or travel from CSL Behring and Novartis. GS has received grants or contracts from Alnylam and Pfizer (study funding paid to his institution). GS has received honoraria for lectures from Alnylam. GS has received honoraria from Alnylam for participation to board. JS has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Pfizer. JS has participated on a Data Safety Monitoring Board or Advisory Board for Alnylam. CT has received consulting fees from Alnylam and Pfizer. CT has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Alnylam and Pfizer. CT has received support for attending meetings and/or travel from Alnylam and Pfizer. CT has participated on a Data Safety Monitoring Board or Advisory Board for Alnylam and Pfizer. CH is an employee of Alnylam. YP has received consulting fees from Alnylam. YP has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events. YP has received support for attending meetings and/or travel from Alnylam. YP has participated on a Data Safety Monitoring Board or Advisory Board for Alnylam. JPC and AS declare no conflicts of interest.
Figures
References
-
- Fargeot G, Gitiaux C, Magy L, Pereon Y, Delmont E, Viala K, et al. French recommendations for the management of adult & pediatric chronic inflammatory demyeliniating polyradiculoneuropathy (CIDP). Rev Neurol (Paris). 2022;178:953–68. - PubMed
-
- Hughes R. Chronic inflammatory demyelinating polyradiculoneuropathy. J Clin Immunol. 2010;30(Suppl 1):S70–73. - PubMed
-
- Van den Bergh PYK, van Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force-Second revision. Eur J Neurol. 2021;28:3556–83. - PubMed
-
- Lewis RA, van Doorn PA, Sommer C. Tips in navigating the diagnostic complexities of chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci. 2022;443:120478. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous