Virtual simulation for flow-diverter selection and sizing in the endovascular treatment of intracranial aneurysms: A systematic review and meta-analysis
- PMID: 40025753
- PMCID: PMC11873847
- DOI: 10.1177/15910199251323006
Virtual simulation for flow-diverter selection and sizing in the endovascular treatment of intracranial aneurysms: A systematic review and meta-analysis
Abstract
Background: Endovascular treatment (EVT) of intracranial aneurysms (IAs) has improved significantly with the integration of virtual simulation software (VSS) in surgical planning and device selection. Despite promising outcomes, discrepancies remain between physician and VSS recommendations. This review synthesizes evidence on (1) comparisons between VSS-chosen and physician-chosen dimensions; (2) VSS-chosen and postoperative measured dimensions; and (3) the success rate of VSS-guided device deployment.
Methods: A systematic search adhering to PRISMA guidelines was conducted in Medline, Embase, Web of Science, and Cochrane databases up to January 2024. Eligible studies included case series, cohort studies, and randomized trials assessing VSS for stent selection in IAs treatment. Mean difference (MD) and single-arm meta-analysis with 95% confidence intervals (CIs) under a random-effects model were performed for continuous and binary outcomes. Subanalyses were conducted for Sim&Size and PreSize software.
Results: Ten studies comprising 658 IAs were included. Pipeline Embolization Device was most commonly used. Findings demonstrated (1) high accuracy of VSS when comparing simulated and postoperative lengths (MD -1.7 mm; 95% CI -4.37 to 0.98 mm); (2) physician-chosen lengths overestimated compared to VSS (MD -2.11 mm; -3.43 to -0.79 mm); (3) no significant difference in physician- versus VSS-chosen diameters (MD -0.04 mm; -0.13 to 0.06 mm); and (4) high VSS-guided deployment success (96%; 93-99%) with low complications (4%). Subanalyses showed 95% and 92% deployment success rates for Sim&Size and PreSize, respectively.
Conclusion: VSS effectively estimates device length and achieves high deployment success, with low complication rates, supporting its utility in EVT planning.
Keywords: Flow-diverter; flow-diverter sizing; simulation.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


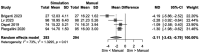




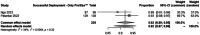
References
-
- Piergallini L, Cagnazzo F, Conte G, et al. Virtual simulation with Sim&Size software for Pipeline Flex Embolization: evaluation of the technical and clinical impact. J Neurointerv Surg 2020; 12: 968–973. - PubMed
-
- Cagnazzo F, Marnat G, Ferreira I, et al. Comparison of Woven EndoBridge device sizing with conventional measurements and virtual simulation using the Sim&Size software: a multicenter experience. J Neurointerv Surg 2021; 13: 924–929. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

