Nutraceutical Evaluation of Trigonelline's Therapeutic Potential by Targeting Bladder Cancer Stem Cells and Cancer-Associated Fibroblasts via Downregulation of TGFβ3/GLI2/YAP1 Signaling Hub
- PMID: 40027190
- PMCID: PMC11866525
- DOI: 10.7150/ijms.107228
Nutraceutical Evaluation of Trigonelline's Therapeutic Potential by Targeting Bladder Cancer Stem Cells and Cancer-Associated Fibroblasts via Downregulation of TGFβ3/GLI2/YAP1 Signaling Hub
Abstract
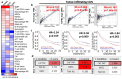
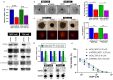
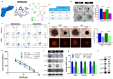
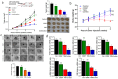
Trigonelline (TGN), an alkaloid identified in medicinal plants such as coffee (Coffea spp.) and fenugreek (Trigonella foenum-graecum), has demonstrated significant anticancer properties across various malignancies, yet its efficacy in bladder cancer (BLCA) remains underappreciated. This study investigates TGN's role in modulating cancer stem cells (CSCs) and the tumor microenvironment (TME), two key contributors to BLCA progression and chemoresistance. Through comprehensive bioinformatics analyses of BLCA patient datasets, a TGY signature (TGFβ3, GLI2, YAP1) was identified as a critical signaling hub associated with poor prognosis, therapeutic resistance, and CSC generation. Computational docking studies revealed TGN's high binding affinity to the TGY signature, TGFβ3 (ΔG = -3.9 kcal/mol), GLI2 (ΔG = -4.2 kcal/mol), YAP1 (ΔG = -3.4 kcal/mol), suggesting its potential to disrupt this signaling axis. In vitro experiments demonstrated that TGN effectively inhibited BLCA cell proliferation, colony formation, and tumorspheroid growth while significantly enhancing cisplatin sensitivity in resistant cell lines. Notably, TGN reduced the transformation of fibroblasts into cancer-associated fibroblasts (CAFs) through the downregulation of α-SMA and FAP (Fibroblast activation protein) expression, indicating its capacity to normalize the TME. Real-time PCR analysis revealed that TGN treatment significantly reduced markers of epithelial-mesenchymal transition and stemness pathways. Our preclinical mouse study demonstrated that combining TGN and cisplatin significantly reduced tumorigenesis in cisplatin-resistant bladder tumoroids harboring CAFs. Importantly, this combination therapy showed no apparent systematic toxicity, suggesting a favorable safety profile. Our findings reveal novel molecular targets of TGN in bladder cancer; TGN acts as a potent disruptor of the TGY signaling axis and a normalizer of the TME by reducing CAF transformation. In sum, our findings advocate for TGN's further exploration as a candidate for combination therapy in drug-resistant BLCA, with the potential to improve patient outcomes by simultaneously targeting both CSCs and the TME, serving as a foundation for future clinical trials.
Keywords: TGFβ3/GLI2/YAP1 signaling; bladder cancer; cancer stem cells; cancer-associated fibroblasts; cisplatin resistance; trigonelline.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Grivas P, Yu EY. Role of Targeted Therapies in Management of Metastatic Urothelial Cancer in the Era of Immunotherapy. Curr Treat Options Oncol. 2019;20:67. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

