Liquid biopsy in gastric cancer: A snapshot of the current state of the art
- PMID: 40027230
- PMCID: PMC11863821
- DOI: 10.1016/j.jlb.2025.100288
Liquid biopsy in gastric cancer: A snapshot of the current state of the art
Abstract
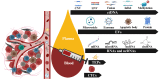
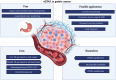
Circulating tumor DNA (ctDNA) is nowadays considered a robust source to search for druggable tumoral genetic alterations, and in some specific settings liquid biopsy (LB) is already part of the diagnostics scenario and it has successfully implemented in the everyday practice. Three strengths make LB an extraordinary tool: i) to represent the complex molecular mosaicism that characterizes spatially heterogeneous malignancies; ii) to monitor in real-time the tumoral molecular landscape (i.e. to depict the longitudinal/temporal tumor evolution); iii) to ensure molecular profiling even in those cases in which tissue sampling is not feasible or not adequate. This review provides a snapshot of the current state of the art concerning ctDNA assay utility in gastric cancer (GC), testing its robustness as marker and seeking to understand the reasons for the delay in its application in clinical practice.
Keywords: CNAs; Circulating markers; Gastric cancer; Liquid biopsy; ctDNA.
© 2025 The Authors.
Conflict of interest statement
I report research funding (to institution) from Roche, Astellas, and Diaceutics; personal honoraria as an invited speaker from Roche, Astellas, AstraZeneca, Lilly, Incyte, Bristol Myers Squibb, Agilent, Merck Serono, Pierre Fabre, GlaxoSmithKline, Novartis, and Amgen; and for participation in advisory boards of Amgen, Astellas, Roche, Merck Serono, GlaxoSmithKline, Novartis, and Janssen.
Figures


References
-
- Rajdev L., Kennedy E.B., Shah M.A. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline Q and A. JCO Oncol Pract. 2023;19(4):197–200. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

