Circulating tumor DNA in clinical trials for solid tumors: Challenges and current applications
- PMID: 40027283
- PMCID: PMC11863815
- DOI: 10.1016/j.jlb.2023.100007
Circulating tumor DNA in clinical trials for solid tumors: Challenges and current applications
Abstract
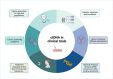
Tumor derived biomarkers including circulating tumor DNA (ctDNA) and/or circulating tumors cells (CTCs) may be detected and quantified through liquid biopsy (LB). ctDNA analysis through LB is a validated tool for monitoring response to systemic treatment and detecting molecular mechanisms of resistance at the time of progression of advanced stage malignancies. Several applications of ctDNA have been investigated in the diagnostic phase of cancer or in the post-curative treatment surveillance phase (e.g., minimal residual disease assessment after neoadjuvant or adjuvant therapy). Recently, the improvement of ctDNA technology and its implementation have affected early phase trials design, with significant changes in the inclusion and randomization phases. Implementation of LB has resulted in large-scale development of academic programs aimed at exploiting all the potential applications of ctDNA, such as patients extended molecular screening, molecular oriented treatment decision making, monitoring of anti-cancer treatments response. In this rapid evolving field, the challenge is no longer the technique, but the evaluation of the results and the interpretation of their impact on diagnosis, prognosis, or therapeutic decision. Leading research cancer centers may favor education for scientific community, by capturing data on this evolving technology and sharing knowledge. In this review we summarize the main applications and challenges of ctDNA genotyping in clinical trials, with special focus on ongoing studies. We finally describe the most important next generation academic and industry-sponsored programs addressing early cancer detection and prevention in high-risk populations through ctDNA genotyping.
Keywords: Cancer; Clinical trials; Early detection; Liquid biopsy; Minimal residual disease; Molecular genotyping; Molecular resistance; ctDNA.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Kalemkerian G.P., Narula N., Kennedy E.B. Molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement summary of the college of American pathologists/international association for. J Oncol Pract. 2018;14(5):323–327. doi: 10.1200/JOP.18.00035. - DOI - PubMed
-
- Mosele F., Remon J., Mateo J., et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(11):1491–1505. doi: 10.1016/j.annonc.2020.07.014. - DOI - PubMed
-
- Flaherty K.T., Gray R.J., Chen A.P., et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer Institute molecular analysis for therapy choice (NCI-MATCH) J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(33):3883–3894. doi: 10.1200/JCO.19.03010. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources