Circulating tumor DNA fraction predicts residual cancer burden post-neoadjuvant chemotherapy in triple negative breast cancer
- PMID: 40027305
- PMCID: PMC11863946
- DOI: 10.1016/j.jlb.2024.100168
Circulating tumor DNA fraction predicts residual cancer burden post-neoadjuvant chemotherapy in triple negative breast cancer
Abstract
Purpose: Pathologic response after preoperative/neoadjuvant chemotherapy (NAC) is a continuum that can range from complete pathologic response (pCR) to extensive residual disease (RD). We hypothesized that post-NAC plasma circulating tumor DNA (ctDNA) fraction (TF) reflect pathologic response as continuum measured by the residual cancer burden (RCB) score.
Methods: ctDNA was assessed using the PredicineBEACON assay, that interrogates up to 50 personalized tumor variants and 500 hot-spot mutations, in 3 mL archived plasma isolated from EDTA tubes collected post-NAC but before surgery from 44 patients with stage I/III triple negative breast cancer (TNBC) who received durvalumab and weekly nab-paclitaxel followed by doxorubicin/cyclophosphamide on a clinical trial (NCT02489448). Circulating free tumor DNA methylation profiling was performed using PredicineEPIC assay in paired pre- and post-NAC plasma (N = 30). Youden's J-statistics was used to define optimal thresholds.
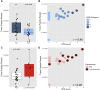
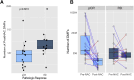
Results: We observed a significant positive correlation (r = 0.45, p = 0.004) between RCB scores and post-NAC TF. The median TF was significantly lower in pCR (RCB0) compared to RD patients (0.06 % vs. 0.3 %, p = 0.02). Using a TF positivity threshold of ≥0.05 %, PredicineBEACON had 58 % sensitivity at 83 % specificity for identifying RD. TF was higher in patients who experienced recurrence (n = 9) compared to those without recurrence (n = 35) (0.17 % vs. 0.05 % TF, p = 0.029). There was significant decrease in methylation signal in post-compared to pre-NAC samples, but post-treatment methylation signal was lower in cases with pCR vs RD.
Conclusions: Post-NAC plasma tumor fraction and change in tumor-derived methylation signal predict the extent of RD and recurrence in TNBC patients.
Keywords: Circulating tumor DNA (ctDNA); Minimal residual disease (MRD); Residual cancer burden (RCB).
© 2024 The Authors.
Conflict of interest statement
NLS, GEC, KB, JF have no relevant financial or non-financial interests to disclose. Billie Gould: employment—Predicine Inc; stock—Predicine, Freenome, and Myriad. Pan Du: employment—Predicine, Inc; stock: Predicine, Inc. Myles Walsh: employment—Predicine, Inc; stock: Predicine, Inc. Giancarlo Bonara: employment—Predicine Inc, stock: Predicine, Inc. Xiaohong Wang: employment—Predicine Inc, stock: Predicine, Inc. Lajos Pusztai: has received consulting fees and honoraria for advisory board participation from Pfizer, Astra Zeneca, Merck, Novartis, Bristol-Myers Squibb, Stemline-Menarini, GlaxoSmithKline, Genentech/Roche, Personalis, Daiichi, Natera, Exact Sciences and institutional research funding from Seagen, GlaxoSmithKline, AstraZeneca, Merck, Pfizer and Bristol Myers Squibb.
Figures


References
-
- Yau C., Osdoit M., van der Noordaa M., Shad S., Wei J., de Croze D., et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–160. doi: 10.1016/s1470-2045(21)00589-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

