Synergistic restoration of spinal cord injury through hyaluronic acid conjugated hydrogel-polydopamine nanoparticles combined with human mesenchymal stem cell transplantation
- PMID: 40027446
- PMCID: PMC11871414
- DOI: 10.1016/j.bioactmat.2024.09.027
Synergistic restoration of spinal cord injury through hyaluronic acid conjugated hydrogel-polydopamine nanoparticles combined with human mesenchymal stem cell transplantation
Abstract
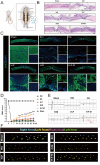
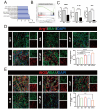
Spinal cord injury (SCI) is a devastating disease with limited treatment options due to the restricted regenerative capacity of the central nervous system. The accumulation of reactive oxygen species (ROS) and inadequate endogenous neural stem progenitor cells (eNSPCs) in the lesion site exacerbates neurologic deficits and impedes motor function recovery. We have developed a temperature-responsive hyaluronic acid conjugated hydrogel-polydopamine nanoparticles (PDA NPs) combined with human mesenchymal stem cell (hMSCs) transplantation, denoted as H-P-M hydrogel. Microglia cells treated with PDA NPs have been shown to reduce intracellular ROS levels by 65 % and suppress the expression of inflammatory cytokines such as IL-1β (decreased by 35 %) and IL-6 (decreased by 23 %), thus mitigating the microglia's inflammatory response. Additionally, our results have demonstrated that the H-P-M hydrogel combined with hMSCs transplantation can recruit eNSPCs to the injury site as evidenced by utilizing Nestin lineage tracer mice. The RNA-seq has unveiled the potential of the H-P-M hydrogel to facilitate eNSPCs neuronal differentiation through the MAPK pathway. Furthermore, these differentiated neurons are integrated into local neural circuits. Together, it suggests that the H-P-M hydrogel synergistically improves the SCI niche. It serves as catalysts inducing 5-HT axon regeneration and improving BMS score after SCI through the modulation of the ROS milieu and the promotion of neuronal differentiation from eNSPCs, thereby presenting a promising strategy for SCI repair.
Keywords: Endogenous neural stem progenitor cells; Human mesenchymal stem cells; Polydopamine nanoparticles; Spinal cord injury.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources

