Multi-omics unveils strain-specific neuroactive metabolite production linked to inflammation modulation by Bacteroides and their extracellular vesicles
- PMID: 40027450
- PMCID: PMC11868947
- DOI: 10.1016/j.crmicr.2025.100358
Multi-omics unveils strain-specific neuroactive metabolite production linked to inflammation modulation by Bacteroides and their extracellular vesicles
Abstract
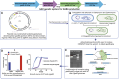
Bacteroides species are key members of the human gut microbiome and play crucial roles in gut ecology, metabolism, and host-microbe interactions. This study investigated the strain-specific production of neuroactive metabolites by 18 Bacteroidetes (12 Bacteroides, 4 Phocaeicola, and 2 Parabacteroides) using multi-omics approaches. Genomic analysis revealed a significant potential for producing GABA, tryptophan, tyrosine, and histidine metabolism-linked neuroactive compounds. Using untargeted and targeted metabolomics, we identified key neurotransmitter-related or precursor metabolites, including GABA, l-tryptophan, 5-HTP, normelatonin, kynurenic acid, l-tyrosine, and norepinephrine, in a strain- and media-specific manner, with GABA (1-2 mM) being the most abundant. Additionally, extracellular vesicles (EVs) produced by Bacteroides harbor multiple neuroactive metabolites, mainly GABA, and related key enzymes. We used CRISPR/Cas12a-based gene engineering to create a knockout mutant lacking the glutamate decarboxylase gene (gadB) to demonstrate the specific contribution of Bacteroides finegoldii-derived GABA in modulating intestinal homeostasis. Cell-free supernatants from wild-type (WT, GABA+) and ΔgadB (GABA-) provided GABA-independent reinforcement of epithelial membrane integrity in LPS-treated Caco-2/HT29-MTX co-cultures. EVs from WT and ΔgadB attenuated inflammatory immune response of LPS-treated RAW264.7 macrophages, with reduced pro-inflammatory cytokines (IL-1β and IL-6), downregulation of TNF-α, and upregulation of IL-10 and TGF-β. GABA production by B. finegoldii had a limited impact on gut barrier integrity but a significant role in modulating inflammation. This study is the first to demonstrate the presence of a myriad of neuroactive metabolites produced by Bacteroides species in a strain- and media-specific manner in supernatant and EVs, with GABA being the most dominant metabolite and influencing immune responses.
Keywords: Bacteroides; Extracellular vesicles; GABA; Gut microbiome; Immunomodulation; Multi-omics; Neuroactive metabolites.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Riadh Hammami reports financial support was provided by Natural Sciences and Engineering Research Council of Canada (NSERC). NSERC had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Yousuf B, Mottawea W, and Hammami R are listed as inventors on a pending patent application #GUT MICROBIOME PSYCHOBIOTICS, EXTRACELLULAR VESICLES THEREFROM, COMPOSITIONS COMPRISING THE SAME AND METHODS OF USING SAME (US Patent Application Number 63/733,497, filled December 13, 2024), assigned to University of Ottawa. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver. Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. - DOI - PubMed
LinkOut - more resources
Full Text Sources

