This is a preprint.
Mitochondrially Transcribed dsRNA Mediates Manganese-induced Neuroinflammation
- PMID: 40027638
- PMCID: PMC11870567
- DOI: 10.1101/2025.02.16.638529
Mitochondrially Transcribed dsRNA Mediates Manganese-induced Neuroinflammation
Abstract
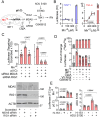
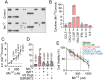
Manganese (Mn) is an essential trace element required for various biological functions, but excessive Mn levels are neurotoxic and lead to significant health concerns. The mechanisms underlying Mn-induced neurotoxicity remain poorly understood. Neuropathological studies of affected brain regions reveal astrogliosis, and neuronal loss, along with evidence of neuroinflammation. Here, we present a novel Mn-dependent mechanism linking mitochondrial dysfunction to neuroinflammation. We found that Mn disrupts mitochondrial transcriptome processing, resulting in the accumulation of complementary RNAs that form double-stranded RNA (dsRNA). This dsRNA is released to the cytoplasm, where it activates cytosolic sensor pathways, triggering type I interferon responses and inflammatory cytokine production. This mechanism is evident in 100-day human cerebral organoids, where Mn-induced inflammatory responses are observed predominantly in mature astrocytes. Similar effects were observed in the transcriptome and cytokine profile of female and male mouse brains carrying mutations in the SLC30A10 gene, a model of hypermanganesemia with dystonia1 disorder. These findings highlight a previously unrecognized role for mitochondrial dsRNA in Mn-induced neuroinflammation and provide insights into the molecular pathogenesis of manganism. We propose that this mitochondrial dsRNA-induced inflammatory pathway could be active in other diseases caused by environmental or genetic factors.
Keywords: astrocyte; dsRNA; interferon; manganese.
Conflict of interest statement
Competing interests The authors report no competing interests.
Figures




References
-
- Schullehner J, Thygesen M, Kristiansen SM, Hansen B, Pedersen CB, Dalsgaard S. Exposure to Manganese in Drinking Water during Childhood and Association with Attention-Deficit Hyperactivity Disorder: A Nationwide Cohort Study. Environ Health Perspect. Sep 2020;128(9):97004. doi: 10.1289/EHP6391 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
