This is a preprint.
Cholesterol efflux protein, ABCA1, supports anti-cancer functions of myeloid immune cells
- PMID: 40027727
- PMCID: PMC11870514
- DOI: 10.1101/2025.02.19.638515
Cholesterol efflux protein, ABCA1, supports anti-cancer functions of myeloid immune cells
Abstract
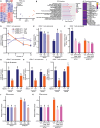
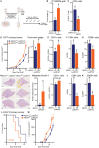
Although immune therapy has seen significant advances, the majority of breast and other solid tumors do not respond or quickly develop de novo resistance. One factor driving resistance is highly immune suppressive myeloid cells (MCs) such as macrophages. Previous work has established clinical links between cholesterol and cancer outcome, and that MC function can be regulated through disruption in cholesterol metabolism. Thus, we screened for proteins that were expressed in MCs, involved in cholesterol homeostasis and whose expression was associated with survival; we identify the cholesterol efflux protein ABCA1. Preclinical studies revealed that ABCA1 activity resulted in increased anti-cancer functions of macrophages: enhanced tumor infiltration, decreased angiogenic potential, reduced efferocytosis, and improved support of CD8+ T cell activity. Mechanistically, different AKT isoforms are involved, through both PI3K dependent and independent mechanisms. Assessment of human blood and breast tumors revealed correlations between ABCA1 in macrophages and angiogenic potential, VEGFA, and CD8 T cell abundance and activity, highlighting the clinical relevance of our findings. The culmination of the effects of ABCA1 on MC function were demonstrated through increased tumor growth and metastasis in mice with MC specific knockout of ABCA1. Therefore, modulating ABCA1 activity within MCs may represent a novel approach to immune therapy.
Conflict of interest statement
Conflict of interests: SVB and ERN have filed an invention disclosure describing the use of ABCA1 and other strategies to alter ABCA1 activity.
Figures
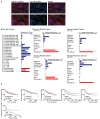


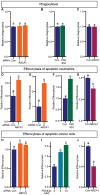
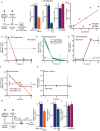
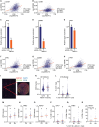
References
-
- Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. The New England journal of medicine. 2022;386(6):556–67. - PubMed
-
- Polk A, Svane IM, Andersson M, and Nielsen D. Checkpoint inhibitors in breast cancer - Current status. Cancer Treat Rev. 2018;63:122–34. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
