This is a preprint.
Comparative molecular landscapes of immature neurons in the mammalian dentate gyrus across species reveal special features in humans
- PMID: 40027814
- PMCID: PMC11870590
- DOI: 10.1101/2025.02.16.638557
Comparative molecular landscapes of immature neurons in the mammalian dentate gyrus across species reveal special features in humans
Update in
-
Cross-species analysis of adult hippocampal neurogenesis reveals human-specific gene expression but convergent biological processes.Nat Neurosci. 2025 Sep;28(9):1820-1829. doi: 10.1038/s41593-025-02027-9. Epub 2025 Aug 11. Nat Neurosci. 2025. PMID: 40790267
Abstract
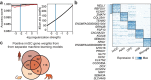
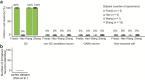
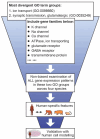
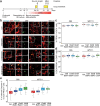
Immature dentate granule cells (imGCs) arising from adult hippocampal neurogenesis contribute to plasticity, learning and memory, but their evolutionary changes across species and specialized features in humans remain poorly understood. Here we performed machine learning-augmented analysis of published single-cell RNA-sequencing datasets and identified macaque imGCs with transcriptome-wide immature neuronal characteristics. Our cross-species comparisons among humans, monkeys, pigs, and mice showed few shared (such as DPYSL5), but mostly species-specific gene expression in imGCs that converged onto common biological processes regulating neuronal development. We further identified human-specific transcriptomic features of imGCs and demonstrated functional roles of human imGC-enriched expression of a family of proton-transporting vacuolar-type ATPase subtypes in development of imGCs derived from human pluripotent stem cells. Our study reveals divergent gene expression patterns but convergent biological processes in the molecular characteristics of imGCs across species, highlighting the importance of conducting independent molecular and functional analyses for adult neurogenesis in different species.
Keywords: adult neurogenesis; cross-species comparison; dentate gyrus; evolution; hippocampus; human-specific feature; immature neuron; machine learning; non-human primate; single-cell RNA sequencing.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures









References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
