Type II Toxin-Antitoxin Systems in Escherichia coli
- PMID: 40027916
- PMCID: PMC11869752
- DOI: 10.2147/IDR.S501485
Type II Toxin-Antitoxin Systems in Escherichia coli
Abstract
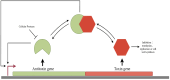
The toxin-antitoxin (TA) system is widespread in prokaryotes and archaea, comprising toxins and antitoxins that counterbalance each other. Based on the nature and mode of action of antitoxins, they are classified into eight groups (type I to VIII). Both the toxins and the antitoxins are proteins in type II TA systems, and the antitoxin gene is usually upstream of the toxin gene. Both genes are organized in an operon and expression of which is regulated at the transcriptional level by the antitoxin-toxin complex, which binds the operon DNA through the DNA-binding domain of the antitoxin. The TA system plays a crucial role in various cellular processes, such as programmed cell death, cell growth, persistence, and virulence. Currently, Type II TA systems have been used as a target for developing new antibacterial agents for treatment. Therefore, the focus of this review is to understand the unique response of Type II TA in Escherichia coli to stress and its contribution to the maintenance of resistant strains. Here, we review the Type II TA system in E. coli and describe their regulatory mechanisms and biological functions. Understanding how TA promotes phenotypic heterogeneity and pathogenesis mechanisms may help to develop new treatments for infections caused by pathogens rationally.
Keywords: E. coli; bacterial persistence; biofilm formation; phage infection; type II toxin-antitoxin.
© 2025 Zhang et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

