Asymmetric Synthesis of Functionalized 2-Isoxazolines
- PMID: 40028108
- PMCID: PMC11866192
- DOI: 10.1021/acsomega.4c08062
Asymmetric Synthesis of Functionalized 2-Isoxazolines
Abstract
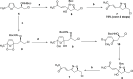
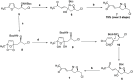
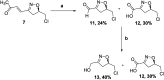
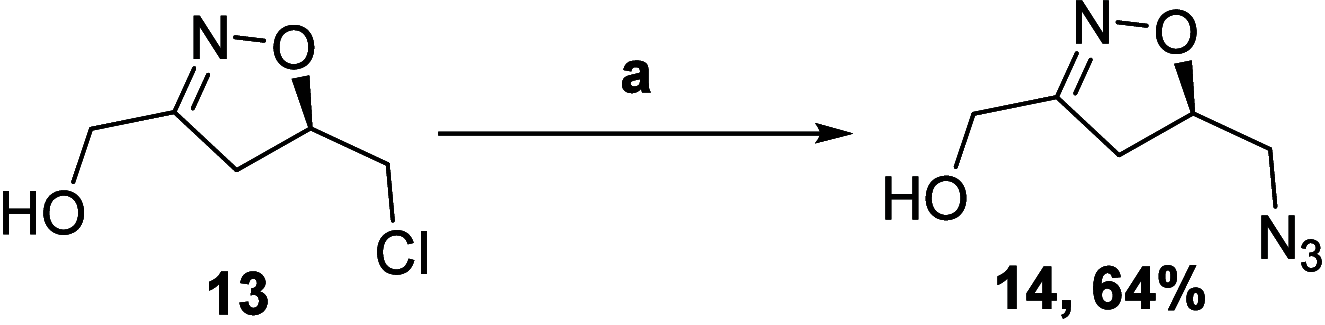
The asymmetric synthesis of the isoxazoline skeleton was achieved by the oxidation of hydroxylaminoalkyl furan prepared from simple starting materials: 2-methylfuran and (S)-epichlorohydrin. The synthesis features an NBS-mediated oxidation of hydroxylaminoalkyl furan to give an α,β-unsaturated ketone intermediate which cyclized to the isoxazoline. The α,β-unsaturated double bond was successfully cleaved with RuCl3·xH2O catalysis en route to the isoxazoline skeleton bearing alcohol, aldehyde, and carboxylic acid functionalities.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

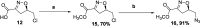
References
-
- Mirjafary Z.; Abdoli M.; Saeidian H.; Kakanejadifard A.; Farnia S. M. F. Review of the Synthesis of Acyclic and Cyclic Oxime Ethers. RSC Adv. 2016, 6, 17740–17758. 10.1039/C5RA25591K. - DOI
-
- Lamberth C. Oxazole and Isoxazole Chemistry in Crop Protection. J. Heterocycl. Chem. 2018, 55, 2035–2045. 10.1002/jhet.3252. - DOI
-
- Poutiainen P. K.; Palvimo J. J.; Hinkkanen A. E.; Valkonen A.; Väisänen T. K.; Laatikainen R.; Pulkkinen J. T. Discovery of 5-Benzyl-3-Phenyl-4,5-Dihydroisoxazoles and 5-Benzyl-3-Phenyl-1,4,2-Dioxazoles as Potent Firefly Luciferase Inhibitors. J. Med. Chem. 2013, 56, 1064–1073. 10.1021/jm301516q. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
