Ultrasensitive Assays Detect Different Conformations of Plasma β Amyloids
- PMID: 40028141
- PMCID: PMC11865983
- DOI: 10.1021/acsomega.4c10879
Ultrasensitive Assays Detect Different Conformations of Plasma β Amyloids
Abstract
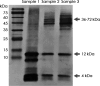
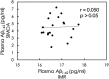
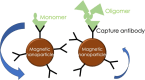
With the developments of ultrasensitive technologies such as immunomagnetic reduction (IMR) assay, single molecule array (SIMOA) assay, electrochemiluminescence immunoassay (ECLIA), the assay of blood-based amyloid 1-42 (Aβ1-42) becomes possible. However, the changes in measured plasma Aβ1-42 concentrations in Alzheimer's disease (AD) compared to cognitively unimpaired subjects (CU) are inconsistent. A possible reason for the inconsistency regarding various conformations of Aβ1-42 in plasma is explored in this study. Three samples with equal amounts of Aβ1-42 but different proportions of monomers and oligomers of Aβ1-42 were prepared. The Aβ1-42 composition of monomers and oligomers in samples was analyzed with Western blot. Identically diluted versions of these three samples were assayed with IMR and SIMOA for Aβ1-42 concentrations. The three diluted samples showed similar levels of Aβ1-42 assayed with IMR, whereas much lower levels for samples with more oligomers assayed with SIOMA. The results imply that IMR detects both monomers and oligomers of Aβ1-42. The measured levels of Aβ1-42 are independent of the proportions of monomer or oligomer Aβ1-42 but depend on the total amounts of Aβ1-42. In the case of SIMOA, monomers of Aβ1-42 are the primary target measured. By comparing Aβ1-42 concentrations of the plasma using IMR and SIMOA, the significant difference in plasma Aβ1-42 levels using IMR in AD compared to CU is mainly due to the formations of oligomeric Aβ1-42. Therefore, if the target molecules are monomers of Aβ1-42, SIMOA is the method of choice. Still, if the target molecules should include monomers, small and large oligomers, IMR would be an optimal consideration. In the future, the clinical implications of the proportion of oligomeric Aβ1-42 need to be elucidated.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Comparison of ELISA- and SIMOA-based quantification of plasma Aβ ratios for early detection of cerebral amyloidosis.Alzheimers Res Ther. 2020 Dec 5;12(1):162. doi: 10.1186/s13195-020-00728-w. Alzheimers Res Ther. 2020. PMID: 33278904 Free PMC article.
-
Long-Term Storage Effects on Stability of Aβ1-40, Aβ1-42, and Total Tau Proteins in Human Plasma Samples Measured with Immunomagnetic Reduction Assays.Dement Geriatr Cogn Dis Extra. 2019 Feb 12;9(1):77-86. doi: 10.1159/000496099. eCollection 2019 Jan-Apr. Dement Geriatr Cogn Dis Extra. 2019. PMID: 31043966 Free PMC article.
-
Plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL across the Alzheimer's disease continuum: A cross-sectional and longitudinal study in the AIBL cohort.Alzheimers Dement. 2023 Apr;19(4):1117-1134. doi: 10.1002/alz.12724. Epub 2022 Jul 21. Alzheimers Dement. 2023. PMID: 36574591
-
Immunomagnetic Reduction Detects Plasma Aβ1-42 Levels as a Potential Dominant Indicator Predicting Cognitive Decline.Neurol Ther. 2020 Dec;9(2):435-442. doi: 10.1007/s40120-020-00215-2. Epub 2020 Oct 22. Neurol Ther. 2020. PMID: 33090326 Free PMC article. Review.
-
Biomarkers with Plasma Amyloid β and Tau Protein Assayed by Immunomagnetic Reduction in Patients with Amnestic Mild Cognitive Impairment and Alzheimer's Disease.Acta Neurol Taiwan. 2022 Jun 30;31(2):53-60. Acta Neurol Taiwan. 2022. PMID: 35040113 Review.
References
-
- Coria F.; Prelli F.; Castaño E. M.; Larrondo-Lillo M.; Fernandez-Gonzalez J.; van Duinen S. G.; Bots G. T.; Luyendijk W.; Shelanski M. L.; Frangione B. Beta-protein deposition: a pathogenetic link between Alzheimer’s disease and cerebral amyloid angiopathies. Brain Res. 1988, 463 (1), 187–191. 10.1016/0006-8993(88)90545-8. - DOI - PubMed
-
- Fan L. Y.; Tzen K. Y.; Chen Y. F.; Chen T. F.; Lai Y. M.; Yen R. F.; Huang Y. Y.; Shiue C. Y.; Yang S. Y.; Chiu M. J. The relation between brain amyloid deposition, cortical atrophy, and plasma biomarkers in amnesic mild cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 17510.3389/fnagi.2018.00175. - DOI - PMC - PubMed
-
- Hampel H.; Hardy J.; Blennow K.; Chen C.; Perry G.; Kim S. H.; Villemagne V. L.; Aisen P.; Vendruscolo M.; Iwatsubo T.; Masters C. L.; Cho M.; Lannfelt L.; Cummings J. L.; Vergallo A. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26 (10), 5481–5503. 10.1038/s41380-021-01249-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
