The effect of cannabinoid type Ⅱ receptor on the excitability of substantia nigra dopaminergic neurons
- PMID: 40028168
- PMCID: PMC11867961
- DOI: 10.3389/fphar.2025.1522210
The effect of cannabinoid type Ⅱ receptor on the excitability of substantia nigra dopaminergic neurons
Abstract
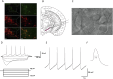
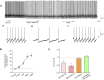
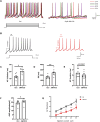
The biological effects of cannabinoids are mainly mediated by two members of the G-protein-coupled-receptor family: cannabinoid type 1 receptor (CB1R) and cannabinoid type 2 receptor (CB2R). Unlike CB1R, CB2R is considered a "peripheral" cannabinoid receptor. However, recent studies have found that CB2R is widely expressed in the central nervous system and is involved in dopamine related behavioral regulation, including dietary behavior, weight regulation, anxiety, and schizophrenia like behavior. Our previous laboratory research demonstrated that activating CB2R on dopaminergic neurons in the ventral tegmental area can regulate addictive behavior in animals by inhibiting neuronal excitability. However, it is currently unclear whether CB2R on dopaminergic neurons in the substantia nigra compacta (SNc) has similar therapeutic potential. Brain patch clamp results have shown that the CB2R agonist JWH133 significantly inhibits the discharge of SNc dopamine neurons in a concentration dependent manner. The pharmacological blocker AM630 of CB2R can reverse this inhibitory effect, indicating that the expression of CB2R in SNc dopaminergic neurons is functional. After treatment with JWH133, the number of induced action potentials decreased, and the peak potential interval time, action potential start time, and potential amplitude after hyperpolarization amplitude all increased. In addition, synaptic current results showed that JWH133 can significantly reduce the frequency of miniature excitatory postsynaptic currents, indicating that activating CB2R to some extent inhibits the release of presynaptic glutamate and indirectly excites postsynaptic neurons.
Keywords: AM630; JWH133; cannabinoid type II receptor; dopamine neurons; firing activity; substantial nigra.
Copyright © 2025 Zhao, Liu, Gong and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mechanisms of cannabinoid CB2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area.EBioMedicine. 2019 Apr;42:225-237. doi: 10.1016/j.ebiom.2019.03.040. Epub 2019 Apr 3. EBioMedicine. 2019. PMID: 30952618 Free PMC article.
-
Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects.Int J Mol Sci. 2020 Dec 21;21(24):9763. doi: 10.3390/ijms21249763. Int J Mol Sci. 2020. PMID: 33371336 Free PMC article.
-
Pharmacological potential of JWH133, a cannabinoid type 2 receptor agonist in neurodegenerative, neurodevelopmental and neuropsychiatric diseases.Eur J Pharmacol. 2021 Oct 15;909:174398. doi: 10.1016/j.ejphar.2021.174398. Epub 2021 Jul 29. Eur J Pharmacol. 2021. PMID: 34332924 Review.
-
Paraquat inhibits postsynaptic AMPA receptors on dopaminergic neurons in the substantia nigra pars compacta.Biochem Pharmacol. 2008 Oct 30;76(9):1155-64. doi: 10.1016/j.bcp.2008.08.006. Epub 2008 Aug 12. Biochem Pharmacol. 2008. PMID: 18761327
-
The electrophysiological actions of dopamine and dopaminergic drugs on neurons of the substantia nigra pars compacta and ventral tegmental area.Life Sci. 1992;51(10):711-8. doi: 10.1016/0024-3205(92)90479-9. Life Sci. 1992. PMID: 1355254 Review.
References
-
- Amancio-Belmont O., Becerril Meléndez A. L., Ruiz-Contreras A. E., Méndez-Díaz M., Prospéro-García O. (2020). Maternal separation plus social isolation during adolescence reprogram brain dopamine and endocannabinoid systems and facilitate alcohol intake in rats. Brain Res. Bull. 164, 21–28. 10.1016/j.brainresbull.2020.08.002 - DOI - PubMed
-
- Aracil-Fernández A., Trigo J. M., García-Gutiérrez M. S., Ortega-Álvaro A., Ternianov A., Navarro D., et al. (2012). Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB₂ receptors. Neuropsychopharmacology 37 (7), 1749–1763. 10.1038/npp.2012.22 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

