NFAT single-deficient murine T cells reduce the risk of aGvHD while controlling cytomegalovirus infection
- PMID: 40028277
- PMCID: PMC11872454
- DOI: 10.1016/j.isci.2025.111937
NFAT single-deficient murine T cells reduce the risk of aGvHD while controlling cytomegalovirus infection
Abstract
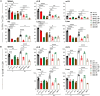
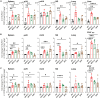
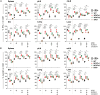
NFAT is a family of transcription factors whose activation is inhibited by calcineurin inhibitors (CNIs). In allogeneic hematopoietic stem cell transplantation (allo-HCT), CNIs are employed to prevent and treat graft-versus-host disease (GvHD). Unfortunately, control of cytomegalovirus (CMV), which exacerbates clinical outcomes, is simultaneously lost. Since single NFAT deficiency in T cells ameliorates GvHD in our major mismatch model, we investigated whether protection is maintained during CMV infection. Reassuringly, NFAT-deficient T cells still improved GvHD upon acute CMV infection and after allo-HCT in latently CMV-infected mice, showing reduced proinflammatory and cytotoxic potential. In sharp contrast, CMV-specific NFAT-deficient CD8+ inflated memory T cells expanded more and with higher levels of interferon gamma (IFN-γ) and GzmB expression, effectively controlling CMV. Notably, NFAT-deficient inflated memory T cells could migrate to non-lymphoid tissues and fight CMV. Therefore, CMV infection does not interfere with the protective effect of NFAT inhibition to attenuate GvHD while allowing an anti-CMV response.
Keywords: Biological sciences; Immunology; Microbiology; Natural sciences; Virology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Insufficient Antigen Presentation Due to Viral Immune Evasion Explains Lethal Cytomegalovirus Organ Disease After Allogeneic Hematopoietic Cell Transplantation.Front Cell Infect Microbiol. 2020 Apr 15;10:157. doi: 10.3389/fcimb.2020.00157. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32351904 Free PMC article.
-
Selective NFAT targeting in T cells ameliorates GvHD while maintaining antitumor activity.Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):1125-30. doi: 10.1073/pnas.1409290112. Epub 2015 Jan 12. Proc Natl Acad Sci U S A. 2015. PMID: 25583478 Free PMC article.
-
Dynamics of cytomegalovirus-specific T-cell recovery in allogeneic hematopoietic cell transplant recipients using a commercially available flow cytometry assay: A pilot study.Transpl Infect Dis. 2024 Jun;26(3):e14290. doi: 10.1111/tid.14290. Epub 2024 May 6. Transpl Infect Dis. 2024. PMID: 38708941
-
Cytomegalovirus immune evasion sets the functional avidity threshold for protection by CD8 T cells.Med Microbiol Immunol. 2023 Apr;212(2):153-163. doi: 10.1007/s00430-022-00733-w. Epub 2022 Apr 1. Med Microbiol Immunol. 2023. PMID: 35364731 Free PMC article. Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
References
-
- Vaeth M., Bäuerlein C.A., Pusch T., Findeis J., Chopra M., Mottok A., Rosenwald A., Beilhack A., Berberich-Siebelt F. Selective NFAT targeting in T cells ameliorates GvHD while maintaining antitumor activity. Proc. Natl. Acad. Sci. USA. 2015;112:1125–1130. doi: 10.1073/pnas.1409290112. - DOI - PMC - PubMed
-
- Vaeth M., Schliesser U., Müller G., Reissig S., Satoh K., Tuettenberg A., Jonuleit H., Waisman A., Müller M.R., Serfling E., et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2012;109:16258–16263. doi: 10.1073/pnas.1203870109. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

