Factor B as a therapeutic target for the treatment of complement-mediated diseases
- PMID: 40028332
- PMCID: PMC11868072
- DOI: 10.3389/fimmu.2025.1537974
Factor B as a therapeutic target for the treatment of complement-mediated diseases
Abstract
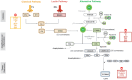
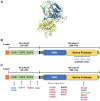
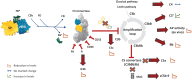
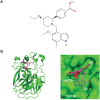
The complement system, consisting of three initiating pathways-classical, lectin and alternative, is an important part of innate immunity. Dysregulation of the complement system is implicated in the pathogenesis of several autoimmune and inflammatory diseases. Therapeutic inhibition of the complement system has been recognized as a viable approach to drug development and has been successful with the approval of a small number of complement inhibitors for diseases such as paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, neuromyelitis optica, myasthenia gravis and geographic atrophy. More recently, therapies selectively targeting the alternative pathway (AP), which drives the amplification of the complement responses, are being evaluated for these complement-mediated diseases. Complement Factor B, a serine protease, is a unique component of the AP that is essential for the catalytic activity of AP C3 convertase and AP C5 convertase. Inhibition of Factor B blocks the activity of the alternative pathway and the amplification loop, and subsequent generation of the membrane attack complex downstream; however, it has no effect on the initial activation mediated by the classical and lectin complement pathways. Therefore, Factor B is an attractive target for diseases in which the AP is overactivated. In this review, we provide an overview of Factor B and its critical role in the AP, discuss the benefit-risk of Factor B inhibition as a targeted therapeutic strategy, and describe the various Factor B inhibitors that are approved and/or in clinical development.
Keywords: alternative pathway; complement; factor B; inhibitors; pharmacological treatment; therapeutic target.
Copyright © 2025 Kavanagh, Barratt, Schubart, Webb, Meier and Fakhouri.
Conflict of interest statement
AS is an employee of Novartis. MM and NW were employees of Novartis at the time of manuscript development. JB has received research funding and/or consultancy fees from Alexion, Alnylam, Arrowhead Pharmaceuticals, AstraZeneca, BioCryst, Kira Pharma, Novartis, Omeros, Roche/Genentech, Bio and Vifor. FF has received consultancy fees from Alexion, Apellis, Novartis, Roche, and Sobi. DK was academic founder of Gyroscope Therapeutics Ltd, now a Novartis Company, and has received consultancy income and equity from Gyroscope Therapeutics Ltd. DK has received honoraria for consultancy work from Alexion, Novartis, Sarepta Chemocentryx, Apellis and Idorsia. DK is an author of patent applications referencing recombinant complement factor I production and/or formation of the C3b/FH/FI trimolecular complex.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

