Clinical significance of the tumor microenvironment on immune tolerance in gastric cancer
- PMID: 40028336
- PMCID: PMC11868122
- DOI: 10.3389/fimmu.2025.1532605
Clinical significance of the tumor microenvironment on immune tolerance in gastric cancer
Abstract
In the realm of oncology, the tumor microenvironment (TME)-comprising extracellular matrix components, immune cells, fibroblasts, and endothelial cells-plays a pivotal role in tumorigenesis, progression, and response to therapeutic interventions. Initially, the TME exhibits tumor-suppressive properties that can inhibit malignant transformation. However, as the tumor progresses, various factors induce immune tolerance, resulting in TME behaving in a state that promotes tumor growth and metastasis in later stages. This state of immunosuppression is crucial as it enables TME to change from a role of killing tumor cells to a role of promoting tumor progression. Gastric cancer is a common malignant tumor of the gastrointestinal tract with an alarmingly high mortality rate. While chemotherapy has historically been the cornerstone of treatment, its efficacy in prolonging survival remains limited. The emergence of immunotherapy has opened new therapeutic pathways, yet the challenge of immune tolerance driven by the gastric cancer microenvironment complicates these efforts. This review aims to elucidate the intricate role of the TME in mediating immune tolerance in gastric cancer and to spotlight innovative strategies and clinical trials designed to enhance the efficacy of immunotherapeutic approaches. By providing a comprehensive theoretical framework, this review seeks to advance the understanding and application of immunotherapy in the treatment of gastric cancer, ultimately contributing to improved patient outcomes.
Keywords: gastric cancer; immunosuppression; immunotherapy; metabolize; tumor microenvironment.
Copyright © 2025 He, Guan and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
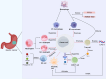
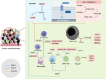
Similar articles
-
Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer.Front Immunol. 2022 Oct 20;13:1016817. doi: 10.3389/fimmu.2022.1016817. eCollection 2022. Front Immunol. 2022. PMID: 36341377 Free PMC article. Review.
-
Gastric cancer immunosuppressive microenvironment heterogeneity: implications for therapy development.Trends Cancer. 2024 Jul;10(7):627-642. doi: 10.1016/j.trecan.2024.03.008. Epub 2024 Apr 9. Trends Cancer. 2024. PMID: 38600020 Free PMC article. Review.
-
Tumor microenvironment: recent advances in understanding and its role in modulating cancer therapies.Med Oncol. 2025 Mar 18;42(4):117. doi: 10.1007/s12032-025-02641-4. Med Oncol. 2025. PMID: 40102282 Review.
-
Role and value of the tumor microenvironment in the progression and treatment resistance of gastric cancer (Review).Oncol Rep. 2025 Jan;53(1):14. doi: 10.3892/or.2024.8847. Epub 2024 Nov 29. Oncol Rep. 2025. PMID: 39611496 Free PMC article. Review.
-
Immune Cell Interactions and Immune Checkpoints in the Tumor Microenvironment of Gastric Cancer.Int J Mol Sci. 2025 Jan 29;26(3):1156. doi: 10.3390/ijms26031156. Int J Mol Sci. 2025. PMID: 39940924 Free PMC article. Review.
Cited by
-
G protein-coupled receptors: pivotal hubs in gastric cancer malignancy-from multidimensional crosstalk to precision therapeutics.J Transl Med. 2025 Aug 7;23(1):879. doi: 10.1186/s12967-025-06851-2. J Transl Med. 2025. PMID: 40775711 Free PMC article. Review.
References
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

