Molecular MRI of T-cell immune response to cryoablation in immunologically hot vs. cold hepatocellular carcinoma
- PMID: 40028344
- PMCID: PMC11870164
- DOI: 10.1016/j.jhepr.2024.101294
Molecular MRI of T-cell immune response to cryoablation in immunologically hot vs. cold hepatocellular carcinoma
Abstract
Background & aims: Increasing enthusiasm around integrating locoregional therapy with systemic immunotherapy in primary liver cancer underscores the need for non-invasive imaging biomarkers. In this study, we aimed to establish advanced molecular MRI tools for monitoring T-cell responses to cryoablation in murine models, distinguishing between immunologically "hot" and "cold" hepatocellular carcinoma (HCC).
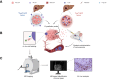
Methods: Immunocompetent 7-10-week-old C57BL/6J and BALB/cJ mice (n = 18 each) received carbon tetrachloride for 12 weeks to induce cirrhosis. Intrinsically immunogenic Hepa1-6 ("hot") and non-immunogenic TiB75 ("cold") cells were orthotopically implanted into C57BL/6 or BALB/c mice, respectively, to generate focal HCC lesions. After one week, animals were randomly assigned to (A) partial cryoablation (pCryo) (1.2 mm cryoprobe, -40 °C) or (B) no treatment (n = 8 per group and tumor type). Gadolinium 160 (160Gd)-labeled CD8+ antibody was administered intravenously either 1 week after tumor induction (control) or 1-week post (pCryo) (treatment). T1-weighted MRI scans were performed using a 9.4 T MRI scanner. Radiological-pathological correlation included imaging mass cytometry and immunohistochemistry.
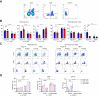
Results: pCryo-treated Hepa1-6 tumors displayed peritumoral ring enhancement on T1-weighted MRI with 160Gd-CD8, correlating with imaging mass cytometry signal patterns. Untreated Hepa1-6 tumors lacked such enhancement. Radiological-pathological correlation confirmed significantly increased tumor-infiltrating CD8+ T lymphocytes in pCryo Hepa1-6 tumors compared with untreated tumors (p <0.001), and a stronger local response compared with systemic lymph nodes (p = 0.0415). Increased T-lymphocyte infiltration was not observed in TiB75 tumors, as indicated by MRI and histopathology.
Conclusion: pCryo induced increased T-cell infiltration in Hepa1-6 tumors compared to TiB75 tumors. T1-weighted MRI, following 160Gd-CD8 antibody administration, reproducibly detected the ablation-induced changes. These findings encourage further investigation of MRI-based molecular imaging biomarkers to assess immune responses to local tumor therapies.
Impact and implications: This study successfully established reliable MR-based molecular imaging tools to visualize CD8+ anti-tumor specific T-cell infiltration following partial cryoablation (pCryo) in murine tumor models. The study's significance lies in advancing our understanding of immune responses within induced cirrhosis and distinguishing between "hot" and "cold" tumor phenotypes. The findings not only build upon previous proof-of-principle data but also extend this technology to include different immune cell types in hepatocellular carcinoma. The study reveals that pCryo may exert specific effects on the tumor microenvironment, augmenting the anti-tumor immune response in immunogenic tumors while displaying a weaker local effect in non-immunogenic tumors.
Keywords: Cryoablation; Immune Response; Immuno-metabolic interplay; In vivo Imaging; Magnetic Resonance Imaging.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICME disclosure forms for further details.
Figures






References
-
- Chen Y.S., Lian L.F., Xu Y.H., et al. [Association of glycosylated hemoglobin level at admission with outcomes of intracerebral hemorrhage patients] Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(11):1445–1449. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

