Human GM-CSF/IL-3 enhance tumor immune infiltration in humanized HCC patient-derived xenografts
- PMID: 40028346
- PMCID: PMC11869099
- DOI: 10.1016/j.jhepr.2024.101264
Human GM-CSF/IL-3 enhance tumor immune infiltration in humanized HCC patient-derived xenografts
Abstract
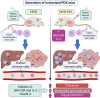
Background & aims: Response to immunotherapy in hepatocellular carcinoma (HCC) is suboptimal with no biomarkers to guide patient selection. "Humanized" mice represent promising models to address this deficiency but are limited by variable chimerism and underdeveloped myeloid compartments. We hypothesized that expression of human GM-CSF and IL-3 increases tumor immune cell infiltration, especially myeloid-derived cells, in humanized HCC patient-derived xenografts.
Material and methods: NOG (NOD/Shi-scid/IL-2Rγnull) and NOG-EXL (huGM-CSF/huIL-3 NOG) mice conditioned with busulfan underwent i.v. injection of human CD34+ cells. HCC patient-derived xenograft tumors were then implanted subcutaneously or orthotopically. Following serial blood sampling, mice were euthanized at defined tumor sizes. Tumor, blood, liver, and spleen were analyzed by flow cytometry and immunohistochemistry.
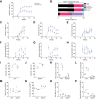
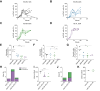
Results: Humanized NOG-EXL mice demonstrated earlier and higher levels of human chimerism compared to humanized NOG mice (82.1% vs. 43.8%, p <0.0001) with a greater proportion of human monocytes (3.2% vs. 1.1%, p = 0.001) and neutrophils (0.8% vs. 0.3%, p = 0.02) in circulation. HCC tumors in humanized NOG-EXL mice exhibited greater human immune cell infiltration (57.6% vs. 30.2%, p = 0.04) with higher proportions of regulatory T cells (14.6% vs. 6.8%, p = 0.04), CD4+ PD-1 expression (84.7% vs. 32.0%, p <0.01), macrophages (1.2% vs. 0.6%, p = 0.02), and neutrophils (0.5% vs. 0.1%, p <0.0001). No differences were observed in tumor engraftment or growth latency in subcutaneous tumors, but orthotopic tumors required implantation at 2 rather than 4 weeks post-humanization for successful engraftment. Finally, utilizing adult bone marrow instead of fetal livers enabled partial HLA-matching to HCC tumors but required more CD34+ cells.
Conclusions: Human GM-CSF and IL-3 expression in humanized mice resulted in features more closely approximating the immune microenvironment of human disease, providing a promising model for investigating critical questions in immunotherapy for HCC.
Impact and implications: This study introduces a unique mouse model at a critical point in the evolution of treatment paradigms for patients with hepatocellular carcinoma (HCC). Immunotherapies have become the first-line treatment for advanced HCC; however, response rates remain low with no clear predictors of response to guide patient selection. In this context, animal models that recapitulate human disease are greatly needed. Leveraging xenograft tumors derived from patients with unresectable HCCs and a commercially available immunodeficient mouse strain that expresses human GM-CSF and IL-3, we demonstrate a novel but accessible approach for modeling the HCC tumor microenvironment.
Keywords: HCC mouse models; humanized mouse; liver cancer; precision medicine; tumor immune microenvironment.
© 2024 Published by Elsevier B.V. on behalf of European Association for the Study of the Liver (EASL).
Conflict of interest statement
KW and DT received research funding from Astra Zeneca through the Society of Interventional Oncology. SJH is a consultant for Boston Scientific, General Electric, and Siemen’s Healthcare. GJN receives research funding from Sirtex Medical, Instylla, and Astra Zeneca. DEK receives research funding from Astra Zeneca, Roche Genetech, Exact Sciences, and Bausch. TPFG is on scientific advisory board for Trisalus Life Sciences. The rest of the authors have declared that no conflict of interest exists. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


Update of
-
Human GM-CSF/IL-3 enhance tumor immune infiltration in humanized HCC patient-derived xenografts.bioRxiv [Preprint]. 2024 Oct 31:2023.10.05.561117. doi: 10.1101/2023.10.05.561117. bioRxiv. 2024. Update in: JHEP Rep. 2024 Nov 08;7(3):101264. doi: 10.1016/j.jhepr.2024.101264. PMID: 39554038 Free PMC article. Updated. Preprint.
References
-
- Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. - PubMed
-
- Abou-Alfa G.K., Lau G., Kudo M., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):1–12. - PubMed
-
- Zhu A.X., Abbas A.R., Ruiz de Galarreta M., et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28:1599–1611. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

