Sensitivity of Cost-Effectiveness to Inclusion of Adverse Drug Events: A Scoping Review of Economic Models of Pharmacological Interventions for Diabetes, Diabetic Retinopathy, and Diabetic Macular Edema
- PMID: 40028500
- PMCID: PMC11872088
- DOI: 10.2147/CEOR.S509349
Sensitivity of Cost-Effectiveness to Inclusion of Adverse Drug Events: A Scoping Review of Economic Models of Pharmacological Interventions for Diabetes, Diabetic Retinopathy, and Diabetic Macular Edema
Abstract
Purpose: Incorporation of adverse drug events (ADEs) is suboptimal in economic evaluation, and thus the information provided by it may be inaccurate. Better guidance on incorporating ADEs into economic evaluation prompts for exploring whether the results are sensitive to ADEs.
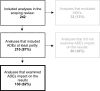
Methods: This scoping review explored 242 cost-effectiveness models for pharmacological interventions for type 1 (T1DM) and 2 diabetes (T2DM), diabetic retinopathy (DR), and diabetic macular edema (DME), in relation to the type of ADEs included in the models (if any), whether the results were sensitive to the ADEs, and what could explain their potential impact.
Results: Of the analyses partly or completely including ADEs, 62% examined their impact on the results, with half of them (50%) reporting ADE-related sensitivity. The models included common to very common ADEs, and some rare but severe ones. The main reasons for excluding ADEs were low incidence (13%) and no reporting in clinical trials (13%). Many analyses reported no reason for the exclusion (53%). The analyses for T1DM and DR or DME included more severe ADEs and reported a higher ADE-related sensitivity compared to the analyses of T2DM (76,2%, 77.8%, and 46.4%, respectively). Higher incidence of ADEs (60,0%) and time trade off method (72,2%) were associated with higher ADE-related sensitivity (72,2%).
Conclusion: Incidence, condition, and the measure of utility were associated with the results being sensitive to ADEs. ADEs are an important outcome for the results of economic evaluation and better guidance on their inclusion and exclusion is needed.
Keywords: adverse event; complications; economic evaluation; modeling.
© 2025 Pesonen and Kankaanpää.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Similar articles
-
Adverse drug events in cost-effectiveness models of pharmacological interventions for diabetes, diabetic retinopathy, and diabetic macular edema: a scoping review.JBI Evid Synth. 2024 Nov 1;22(11):2194-2266. doi: 10.11124/JBIES-23-00511. JBI Evid Synth. 2024. PMID: 39054883 Free PMC article.
-
Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report.J Am Med Inform Assoc. 1998 May-Jun;5(3):305-14. doi: 10.1136/jamia.1998.0050305. J Am Med Inform Assoc. 1998. PMID: 9609500 Free PMC article.
-
Adverse drug events in cost-effectiveness analyses of interventions for diabetic conditions: a scoping review protocol.JBI Evid Synth. 2022 Dec 1;20(12):3058-3066. doi: 10.11124/JBIES-21-00460. JBI Evid Synth. 2022. PMID: 35916006 Free PMC article.
-
Development and Initial Validation of a Patient-Reported Adverse Drug Event Questionnaire.Drug Saf. 2013 Sep;36(9):765-77. doi: 10.1007/s40264-013-0036-8. Drug Saf. 2013. PMID: 23553447 Clinical Trial.
-
Potential Direct Costs of Adverse Drug Events and Possible Cost Savings Achievable by their Prevention in Tuscany, Italy: A Model-Based Analysis.Drug Saf. 2019 Mar;42(3):427-444. doi: 10.1007/s40264-018-0737-0. Drug Saf. 2019. PMID: 30276630
References
-
- Council of Europe. Glossary of terms related to patient and medication safety Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices. World Health Organization. 2005.
-
- Skelly CL, Cassagnol M, Munakomi S. Adverse events. 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558963/. Accessed September 19, 2024.
-
- European Commission Enterprise Directorate-General. A Guideline on Summary of Product Characteristics (SmPC) 2009. Available from: https://www.gmp-compliance.org/files/guidemgr/smpc_guideline_rev2.pdf. Accessed: October 6, 2024.
-
- NIH. The Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Available from: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.p.... Accessed: October 06, 2024.
Publication types
LinkOut - more resources
Full Text Sources