Cellular origins of mucinous ovarian carcinoma
- PMID: 40028669
- PMCID: PMC11985703
- DOI: 10.1002/path.6407
Cellular origins of mucinous ovarian carcinoma
Abstract
Mucinous ovarian carcinoma (MOC) is a rare histotype of epithelial ovarian cancer. Its origins are obscure: while many mucinous tumours in the ovary are metastases from the gastrointestinal tract, MOC can occur as an ovarian primary; however, the cell of origin is not well established. In this review we summarise the pathological, epidemiological, and molecular evidence for the cellular origins of MOC. We propose a model for the origins of the various tumours of the ovary with mucinous differentiation. We distinguish Müllerian from gastrointestinal-type mucinous differentiation. A small proportion of the latter arise from teratoma and a distinct terminology has been proposed. Other gastrointestinal mucinous tumours are associated with Brenner tumours and arise from their associated benign lesions, Walthard nests. The remaining mucinous tumours develop either through mucinous metaplasia in established Müllerian tumours or with even greater plasticity through gastrointestinal metaplasia of epithelial or mesothelial ovarian inclusions. This model remains to be validated and mechanistically understood and we discuss future research directions. © 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: cell of origin; mucinous; ovarian cancer.
© 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Figures
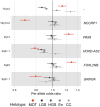
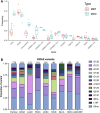
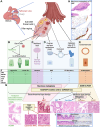
References
-
- Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol 2000; 24: 1447–1464. - PubMed
-
- Talia KL, Parra‐Herran C, McCluggage WG. Ovarian mucinous and seromucinous neoplasms: problematic aspects and modern diagnostic approach. Histopathology 2022; 80: 255–278. - PubMed
-
- McCluggage WG. Immunohistochemistry in the distinction between primary and metastatic ovarian mucinous neoplasms. J Clin Pathol 2012; 65: 596–600. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

