Integrated Efficacy and Safety Analysis of Abrocitinib in Adolescents With Moderate-to-Severe Atopic Dermatitis
- PMID: 40028832
- PMCID: PMC12368748
- DOI: 10.1111/all.16512
Integrated Efficacy and Safety Analysis of Abrocitinib in Adolescents With Moderate-to-Severe Atopic Dermatitis
Abstract
Background: Abrocitinib has demonstrated long-term efficacy (48 weeks) and safety (~4 years) in adults and adolescents with moderate-to-severe atopic dermatitis (AD). This analysis evaluated abrocitinib efficacy in adolescents through 112 weeks, and safety of up to 4.6 years of exposure.
Methods: Data were from adolescents in JADE MONO-1 (NCT03349060), MONO-2 (NCT03575871), TEEN (NCT03796676), REGIMEN (NCT03627767; safety analysis only), and the ongoing phase 3 extension trial, EXTEND (NCT03422822; data cutoff: September 5, 2022). Efficacy assessments included proportions of patients achieving an Investigator's Global Assessment score of 0 or 1 (IGA 0/1) and ≥ 75%/≥ 90% improvement in Eczema Area and Severity Index (EASI-75/-90). Treatment-emergent adverse events (TEAEs) and AEs of special interest were reported as incidence rate/100 patient-years. A substudy of JADE TEEN assessed immune response to vaccination.
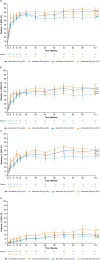
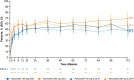
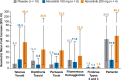
Results: Efficacy was assessed in 170 and 187 patients in the abrocitinib 200-mg and 100-mg arms, respectively; median exposure was 971.0 and 899.0 days. At Week 112, comparable proportions of patients treated with abrocitinib (200, 100 mg) achieved EASI-75 (85%, 83%), EASI-90 (62%, 60%), and IGA 0/1 (57%, 57%). Safety was assessed in 289 and 201 patients in the abrocitinib 200- and 100-mg arms, respectively; median exposure was 882.0 and 863.0 days. Incidence rates were numerically higher with abrocitinib 200 mg versus 100 mg, with overlapping confidence intervals for serious TEAEs (IR [95% CI]; 5.47 [3.69-7.80] vs. 3.45 [1.89-5.80]) and TEAEs leading to discontinuation (6.78 [4.80-9.31] vs. 5.39 [3.38-8.16]).
Conclusions: Efficacy and safety results support long-term abrocitinib use in adolescent patients.
Trail registration: ClinicalTrials.gov Identifiers NCT03349060, NCT03575871, NCT03796676, NCT03627767, NCT03422822.
Keywords: adolescents; atopic dermatitis; efficacy; safety; vaccination.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
A. S. Paller has served as a study investigator for AbbVie, Applied Pharma Research, Dermavant, Eli Lilly and Company, Incyte, Janssen, Krystal Biotech, Regeneron Pharmaceuticals, Timber, and UCB; has served as a consultant for AbbVie, Abeona Therapeutics, Apogee Therapeutics, Arcutis Biotherapeutics, ASLAN Pharma, BioCryst Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Incyte, Johnson & Johnson, Krystal Biotech, LEO Pharma, Mitsubishi Tanabe, Nektar, Primus, Procter & Gamble, Regeneron Pharmaceuticals, Sanofi Genzyme, Seanergy, TWi Biotech, and UCB; and has served on the Data Safety Monitoring Board for AbbVie, Abeona Therapeutics, and Galderma. L. F. Eichenfield has served as a scientific adviser, consultant, and/or clinical study investigator for Pfizer Inc., AbbVie, Almirall, Amgen, Arena, ASLAN Pharmaceuticals, Dermavant, Eli Lilly and Company, Forté, Galderma, Glenmark, Incyte, LEO Pharma, Novartis, Ortho Dermatologics, Otsuka, Regeneron Pharmaceuticals, and Sanofi Genzyme. A. D. Irvine has served as a consultant for Pfizer Inc., AbbVie, Amgen, Arena, Benevolent AI, Eli Lilly and Company, LEO Pharma, Novartis, Regeneron Pharmaceuticals, and Sanofi Genzyme; and has received honoraria for participation in the speakers' bureau for Pfizer Inc., AbbVie, Eli Lilly and Company, LEO Pharma, Novartis, Regeneron Pharmaceuticals, and Sanofi Genzyme. C. Flohr is chief investigator of the UK National Institute for Health Research‐funded TREAT (ISRCTN15837754) and SOFTER (Clinicaltrials.gov: NCT03270566) trials as well as the UK‐Irish Atopic Eczema Systemic Therapy Register (A‐STAR; ISRCTN11210918) and a principal investigator in the European Union (EU) Horizon 2020‐funded BIOMAP Consortium (
Figures


References
-
- Langan S. M., Irvine A. D., and Weidinger S., “Atopic Dermatitis,” Lancet 396, no. 10247 (2020): 345–360. - PubMed
-
- Wollenberg A., Christen‐Zach S., Taieb A., et al., “ETFAD/EADV Eczema Task Force 2020 Position Paper on Diagnosis and Treatment of Atopic Dermatitis in Adults and Children,” Journal of the European Academy of Dermatology and Venereology 34, no. 12 (2020): 2717–2744. - PubMed
-
- Weidinger S., Beck L. A., Bieber T., Kabashima K., and Irvine A. D., “Atopic Dermatitis,” Nature Reviews: Disease Primers 4, no. 1 (2018): 1. - PubMed
-
- Silverberg J. I., Barbarot S., Gadkari A., et al., “Atopic Dermatitis in the Pediatric Population: A Cross‐Sectional, International Epidemiologic Study,” Annals of Allergy, Asthma & Immunology 126, no. 4 (2021): 417–428.e2. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

