Electrostatics of salt-dependent reentrant phase behaviors highlights diverse roles of ATP in biomolecular condensates
- PMID: 40028898
- PMCID: PMC11875540
- DOI: 10.7554/eLife.100284
Electrostatics of salt-dependent reentrant phase behaviors highlights diverse roles of ATP in biomolecular condensates
Abstract
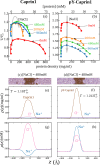
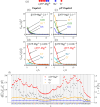
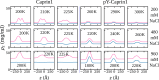
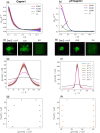
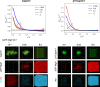
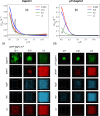
Liquid-liquid phase separation (LLPS) involving intrinsically disordered protein regions (IDRs) is a major physical mechanism for biological membraneless compartmentalization. The multifaceted electrostatic effects in these biomolecular condensates are exemplified here by experimental and theoretical investigations of the different salt- and ATP-dependent LLPSs of an IDR of messenger RNA-regulating protein Caprin1 and its phosphorylated variant pY-Caprin1, exhibiting, for example, reentrant behaviors in some instances but not others. Experimental data are rationalized by physical modeling using analytical theory, molecular dynamics, and polymer field-theoretic simulations, indicating that interchain ion bridges enhance LLPS of polyelectrolytes such as Caprin1 and the high valency of ATP-magnesium is a significant factor for its colocalization with the condensed phases, as similar trends are observed for other IDRs. The electrostatic nature of these features complements ATP's involvement in π-related interactions and as an amphiphilic hydrotrope, underscoring a general role of biomolecular condensates in modulating ion concentrations and its functional ramifications.
Keywords: biochemistry; chemical biology; field-theoretic simulation; intrinsically disordered proteins; liquid-liquid phase separation; molecular biophysics; molecular dynamics; none; phosphorylation; random phase approximation; structural biology.
© 2024, Lin, Kim, Das et al.
Conflict of interest statement
YL, TK, SD, TP, JW, AR, LK, JF, HC No competing interests declared
Figures







Update of
- doi: 10.48550/arXiv.2401.04873
- doi: 10.7554/eLife.100284.1
- doi: 10.7554/eLife.100284.2
References
-
- Alshareedah I, Kaur T, Ngo J, Seppala H, Kounatse L-AD, Wang W, Moosa MM, Banerjee PR. Interplay between short-range attraction and long-range repulsion controls reentrant liquid condensation of ribonucleoprotein-RNA complexes. Journal of the American Chemical Society. 2019;141:14593–14602. doi: 10.1021/jacs.9b03689. - DOI - PMC - PubMed
-
- Anderson JA, Lorenz CD, Travesset A. General purpose molecular dynamics simulations fully implemented on graphics processing units. Journal of Computational Physics. 2008;227:5342–5359. doi: 10.1016/j.jcp.2008.01.047. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

