Stabilization of the catalytically active structure of a molybdenum-dependent formate dehydrogenase depends on a highly conserved lysine residue
- PMID: 40028997
- PMCID: PMC12176258
- DOI: 10.1111/febs.70048
Stabilization of the catalytically active structure of a molybdenum-dependent formate dehydrogenase depends on a highly conserved lysine residue
Abstract
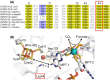
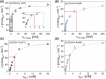
Molybdenum-dependent formate dehydrogenases (Mo-FDHs) reversibly catalyze the interconversion of CO2 and formate, and therefore may be utilized for the development of innovative energy storage and CO2 utilization concepts. Mo-FDHs contain a highly conserved lysine residue in the vicinity of a catalytically active molybdenum (Mo) cofactor and an electron-transferring [4Fe-4S] cluster. In order to elucidate the function of the conserved lysine, we substituted the residue Lys44 of Escherichia coli formate dehydrogenase H (EcFDH-H) with structurally and chemically diverse amino acids. Enzyme kinetic analysis of the purified EcFDH-H variants revealed the Lys-to-Arg substitution as the only amino acid exchange that retained formate oxidation catalytic activity, amounting to 7.1% of the wild-type level. Ultraviolet-visible (UV-Vis) spectroscopic analysis indicated that >90% of the [4Fe-4S] cluster was lost in the case of EcFDH-H variants -K44E and -K44M, whereas the cluster occupancy of the K44R variant decreased by merely 4.5%. Furthermore, the K44R substitution resulted in a slight decrease in its melting temperature and a significant formate affinity decrease, apparent as a 32-fold Km value increase. Consistent with these findings, molecular dynamics simulations predicted an increase in the backbone and cofactor mobility as a result of the K44R substitution. These results are consistent with the conserved lysine being essential for stabilizing the catalytically active structures in EcFDH-H and may support engineering efforts on Mo-FDHs to design more efficient biocatalysts for CO2 reduction.
Keywords: CO2 utilization; lysine; molybdenum‐dependent formate dehydrogenase; redox cofactor; site‐directed mutagenesis.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
In vitro sulfuration of Rhodobacter capsulatus formate dehydrogenase.J Biol Chem. 2025 Jun;301(6):108511. doi: 10.1016/j.jbc.2025.108511. Epub 2025 Apr 15. J Biol Chem. 2025. PMID: 40246024 Free PMC article.
-
A Role for Two Conserved Arginine Residues in Protected Persulfide Transfer by SufE-Dependent SufS Cysteine Desulfurases.Biochemistry. 2025 Jun 3;64(11):2467-2475. doi: 10.1021/acs.biochem.4c00705. Epub 2025 May 21. Biochemistry. 2025. PMID: 40396880 Free PMC article.
-
A growth-based screening strategy for engineering the catalytic activity of an oxygen-sensitive formate dehydrogenase.Appl Environ Microbiol. 2024 Sep 18;90(9):e0147224. doi: 10.1128/aem.01472-24. Epub 2024 Aug 28. Appl Environ Microbiol. 2024. PMID: 39194220 Free PMC article.
-
Molybdenum- and tungsten-containing formate dehydrogenases and formylmethanofuran dehydrogenases: Structure, mechanism, and cofactor insertion.Protein Sci. 2019 Jan;28(1):111-122. doi: 10.1002/pro.3498. Epub 2018 Oct 31. Protein Sci. 2019. PMID: 30120799 Free PMC article.
-
Assembly and catalysis of molybdenum or tungsten-containing formate dehydrogenases from bacteria.Biochim Biophys Acta. 2015 Sep;1854(9):1090-100. doi: 10.1016/j.bbapap.2014.12.006. Epub 2014 Dec 13. Biochim Biophys Acta. 2015. PMID: 25514355 Review.
References
-
- Schwarz G, Mendel RR & Ribbe MW (2009) Molybdenum cofactors, enzymes and pathways. Nature 460, 839–847. - PubMed
-
- Leimkühler S (2020) The biosynthesis of the molybdenum cofactors in Escherichia coli . Environ Microbiol 22, 2007–2026. - PubMed
-
- Mendel RR & Leimkühler S (2015) The biosynthesis of the molybdenum cofactors. J Biol Inorg Chem 20, 337–347. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

