Inflammation and epithelial-mesenchymal transition in a CFTR-depleted human bronchial epithelial cell line revealed by proteomics and human organ-on-a-chip
- PMID: 40029006
- PMCID: PMC12505456
- DOI: 10.1111/febs.70050
Inflammation and epithelial-mesenchymal transition in a CFTR-depleted human bronchial epithelial cell line revealed by proteomics and human organ-on-a-chip
Abstract
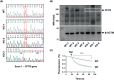
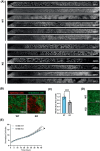
Cystic fibrosis (CF) is a genetic disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, leading to chronic, unresolved inflammation of the airways due to uncontrolled recruitment of polymorphonuclear leukocytes (PMNs). Evidence indicates that CFTR loss-of-function, in addition to promoting a pro-inflammatory phenotype, is associated with an increased risk of developing cancer, suggesting that CFTR can exert tumor-suppressor functions. Three-dimensional (3D) in vitro culture models, such as the CF lung airway-on-a-chip, can be suitable for studying PMN recruitment, as well as events of cancerogenesis, that is epithelial cell invasion and migration, in CF. To gather insight into the pathobiology of CFTR loss-of-function, we generated CFTR-knockout (KO) clones of the 16HBE14o- human bronchial cell line by CRISPR/Cas9 gene editing, and performed a comparative proteomic analysis of these clones with their wild-type (WT) counterparts. Systematic signaling pathway analysis of CFTR-KO clones revealed modulation of inflammation, PMN recruitment, epithelial cell migration, and epithelial-mesenchymal transition. Using a latest-generation organ-on-a-chip microfluidic platform, we confirmed that CFTR-KO enhanced PMN recruitment and epithelial cell invasion of the endothelial layer. Thus, a dysfunctional CFTR affects multiple pathways in the airway epithelium that ultimately contribute to sustained inflammation and cancerogenesis in CF.
Keywords: CRISPR/Cas9; cystic fibrosis; epithelial–mesenchymal transition; organ‐on‐a‐chip; proteomics.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
H.B. and Y.C.Y. are employees of Xellar Inc., which specializes in combining human organ chip models with imaging for disease modeling and drug testing. It should be noted that the devices employed in the research presented in this manuscript are not sourced from Xellar Inc.
Figures




References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073. - PubMed
-
- Cantin AM, Hartl D, Konstan MW & Chmiel JF (2015) Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 14, 419–430. - PubMed
-
- Balázs A & Mall MA (2019) Mucus obstruction and inflammation in early cystic fibrosis lung disease: emerging role of the IL‐1 signaling pathway. Pediatr Pulmonol 54(Suppl 3), S5–S12. - PubMed
MeSH terms
Substances
Grants and funding
- R44 AT002021/AT/NCCIH NIH HHS/United States
- FFC#11/2022 to Domenico Mattoscio/Fondazione per la Ricerca sulla Fibrosi Cistica
- AIRC; #19548 to Tommaso Colangelo/Associazione Italiana per la Ricerca sul Cancro
- Fondazione Umberto Veronesi
- GMSG#01/2023 to Roberto Plebani/Fondazione per la Ricerca sulla Fibrosi Cistica
- R43 AT002021/AT/NCCIH NIH HHS/United States
- U19 AT002022/AT/NCCIH NIH HHS/United States
- MFAG 2022 - ID. 27060 to Domenico Mattoscio/Associazione Italiana per la Ricerca sul Cancro
- AT2022 to Roberto Plebani/Italian Ministry of Health (fund ex60%)
- AT2021 to Roberto Plebani/Italian Ministry of Health (fund ex60%)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

