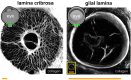
Morphological Comparison of Astrocytes in the Lamina Cribrosa and Glial Lamina
- PMID: 40029245
- PMCID: PMC11887932
- DOI: 10.1167/iovs.66.3.1
Morphological Comparison of Astrocytes in the Lamina Cribrosa and Glial Lamina
Abstract
Purpose: Although the mechanisms underlying glaucomatous neurodegeneration are not yet well understood, cellular and small animal models suggest that lamina cribrosa (LC) astrocytes undergo early morphologic and functional changes, indicating their role as early responders to glaucomatous stress. These models, however, lack the LC found in larger animals and humans, leaving the in situ morphology of LC astrocytes and their role in glaucoma initiation underexplored. In this work, we aimed to characterize the morphology of LC astrocytes in situ and determine differences and similarities with astrocytes in the mouse glial lamina (GL), the analogous structure in a prominent glaucoma model.
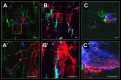
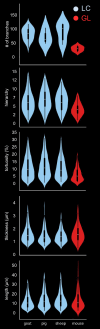
Methods: Astrocytes in the LCs of 22 eyes from goats, sheep, and pigs were stochastically labeled via Multicolor DiOlistics and imaged in situ using confocal microscopy. The 3D models of DiOlistically labeled LC astrocytes and hGFAPpr-GFP mouse GL astrocytes were constructed to quantify morphological features related to astrocyte functions. LC and GL astrocyte cross-pore contacts, branching complexity, branch tortuosity, and cell and branch span were compared.
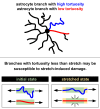
Results: LC astrocytes displayed distinct spatial relationships with collagen, greater branching complexity, and higher branch tortuosity compared to GL astrocytes. Despite substantial differences in their anatomic environments, LC and GL astrocytes had similar cell and branch spans.
Conclusions: Astrocyte morphology in the LC was characterized through multicolor DiOlistic labeling. LC and GL astrocytes have both distinct and shared morphological features. Further research is needed to understand the potentially unique roles of LC astrocytes in glaucoma initiation and progression.
Conflict of interest statement
Disclosure:
Figures






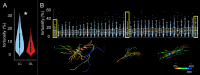
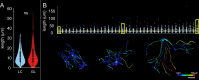
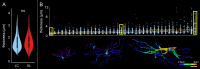

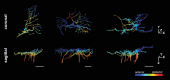
Update of
-
Morphological comparison of astrocytes in the lamina cribrosa and glial lamina.bioRxiv [Preprint]. 2024 Sep 10:2024.09.07.610493. doi: 10.1101/2024.09.07.610493. bioRxiv. 2024. Update in: Invest Ophthalmol Vis Sci. 2025 Mar 03;66(3):1. doi: 10.1167/iovs.66.3.1. PMID: 39314351 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

