Mucilage produced by aerial roots hosts diazotrophs that provide nitrogen in Sorghum bicolor
- PMID: 40029899
- PMCID: PMC12136154
- DOI: 10.1371/journal.pbio.3003037
Mucilage produced by aerial roots hosts diazotrophs that provide nitrogen in Sorghum bicolor
Abstract
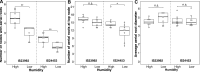
Sorghum (Sorghum bicolor) is an important food, feed, and fodder crop worldwide and is gaining popularity as an energy crop due to its high potential for biomass production. Some sorghum accessions develop many aerial roots and produce an abundant carbohydrate-rich mucilage after rain. This aerial root mucilage is similar to that observed in landraces of maize (Zea mays) from southern Mexico, which have been previously shown to host diazotrophs. In this study, we characterized the aerial root development of several sorghum accessions and the impact of humidity on this trait. We conducted a microbiome study of the aerial root mucilage of maize and sorghum and isolated numerous diazotrophs from field sorghum mucilage. We observed that the prevailing phyla in the mucilage were Pseudomonadota, Bacteroidota, and Bacillota. However, bacterial abundances varied based on the genotype and the location. Using acetylene reduction, 15N2 gas feeding, and 15N isotope dilution assays, we confirmed that these sorghum accessions can acquire about 40% of their nitrogen from the atmosphere through these associations on aerial roots. Nitrogen fixation in sorghum aerial root mucilage offers a promising avenue to reduce reliance on synthetic fertilizers and promote sustainable agricultural practices for food, feed, fodder, and bioenergy production.
Copyright: © 2025 Venado et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Update of
-
Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota.PLoS Biol. 2018 Aug 7;16(8):e2006352. doi: 10.1371/journal.pbio.2006352. eCollection 2018 Aug. PLoS Biol. 2018. Update in: PLoS Biol. 2025 Mar 03;23(3):e3003037. doi: 10.1371/journal.pbio.3003037. PMID: 30086128 Free PMC article. Updated.
References
-
- Burt TP. Nitrogen cycle⋆. In: Fath B, editor. Encyclopedia of ecology. 2nd ed. Oxford: Elsevier; 2013. p. 135–42.
-
- Wagner S. Biological nitrogen fixation. Nature Educ Knowl. 2011;3(15):1–5.
-
- Saikia S, Jain V. Biological nitrogen fixation with non-legumes: an achievable target or a dogma? Curr Sci. 2007;92:317–22.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

